As fuel for most passenger cars gasoline is used. It is a mixture of hydrocarbons with a boiling point of 30 to 205 degrees Celsius. In addition to hydrocarbons, gasoline contains impurities containing nitrogen, sulfur and oxygen.
Depending on the amount of certain compounds, motor gasoline is divided into different brands having slightly different operational properties:
- AI-92;
- AI-95;
- AI-98.
With the tightening of environmental requirements, gasolines with a lower octane number, such as A-76 or AI-80, and, therefore, a more "dirty" chemical composition, are currently not produced.
Basic properties
The main properties of gasoline are its chemical composition, its ability to evaporate, burn, ignite, form deposits, as well as its corrosiveness and resistance to detonation.
The physicochemical properties of gasoline vary depending on what hydrocarbons and in what proportions it contains. The freezing point of gasoline reaches -60 degrees Celsius, if special additives are used, this value can be reduced to -71 degrees. Gasoline actively evaporates at temperatures above 30 degrees, and with an increase in temperature, evaporation is more intense. When the concentration of its vapors in the air reaches 74 - 123 grams per cubic meter, an explosive mixture is formed.

The fractional composition of gasoline directly affects the performance properties. During production, it is important to achieve the correct ratio of light and heavy fractions in order, on the one hand, to ensure a sufficiently high volatility at low temperatures, and on the other hand, to prevent interruptions in the operation of the engine due to the formation of steam locks in the fuel line, which can occur due to intense evaporation. a large number of light fractions. In this regard, gasolines used in hot climates and in the Arctic Circle have a different chemical composition in order to provide the necessary performance properties.
Gasoline can be obtained in several ways: by direct distillation of oil and the selection of certain fractions (this method was used at the beginning of the era of motorization), cracking and reforming began to be used in the middle of the last century. The main constituent of gasoline obtained by direct distillation is alkane chains. During cracking and reforming, they are converted to branched alkanes and aromatics.
The last two methods make it possible to obtain high-octane fuel grades AI-92, 95 and higher.
Octane number
The name of the brand of gasoline consists of an alphanumeric designation. The letters A or AI indicate the method for determining the octane number:
- motor (A)
- research (AI)
and the digit defines the octane number (92, 95, etc.).
The octane number indicates a property such as the knock resistance of gasoline. This figure is relative. Isooctane is taken as a standard, the detonation resistance of which is very high and is taken equal to 100. The octane number scale was proposed at the beginning of the last century. It was determined by the content of isooctane in a mixture with normal heptane (its detonation resistance is very low and is taken to be zero). Accordingly, AI-92 gasoline is equivalent in its resistance to detonation to a 92% mixture of isooctane with heptane, AI-95 - 95%, and so on. The octane number can be more than 100, if the antiknock properties of the fuel are even higher than that of pure isooctane.

This value is very important, since detonation leads to a rapid destruction of the cylinder-piston group. This is explained by the speed of propagation of the flame front - up to 2.5 km / s, while under normal conditions the flame propagates at a speed of no more than 60 m / s.
To increase the anti-knock properties, you can either add additives containing lead compounds (tetraethyl lead), or change the fractional composition upon receipt. The first method is easily obtained from gasoline AI-92 AI-95, or 98, but has now been abandoned. Since, although such additives significantly increase the operational properties of the fuel and have a low cost, they are also very toxic and have a much more destructive effect on the environment than pure gasoline, and also destroy the catalytic converter of a car (the combustion temperature of leaded gasoline is higher than that of unleaded gasoline, as a result, the ceramic elements of the neutralizer are simply sintered, and the device fails).
Other less toxic compounds such as ethyl alcohol or acetone can be used as additives. For example, if you add 100 ml of alcohol to a liter of AI-92 gasoline, the octane number will increase to 95. However, the use of such additives is economically unprofitable.
Chemical stability
Considering the chemical properties of gasoline, the main emphasis should be on how long the composition of hydrocarbons will remain unchanged, since during long-term storage the lighter compounds evaporate and the operational properties deteriorate greatly. This problem is especially acute if a higher grade gasoline (AI-95) was obtained from a fuel with a lower octane number (for example, AI-92) by adding propane or methane to its composition. Their antiknock properties are higher than that of isooctane, but they also evaporate very quickly.
The state standard requires that the chemical composition of gasoline of any brand, be it AI-92, 95 or 98, remain unchanged for at least five years, subject to storage rules. However, in fact, often even just bought fuel already has an octane number lower than the declared one (for example, not 95, but 92). The reason for this is the dishonesty of the sellers who add liquefied gas to the tanks with fuel, the storage period of which has expired, and the composition does not correspond to GOST. As a rule, different amounts of gas are added to the same gasoline to obtain an octane rating of 92 or 95. An obvious confirmation of such tricks is the strong smell of gas at the filling station. It is likely that the performance properties of such gasoline will noticeably deteriorate right before our eyes, until the time the fuel tank is empty.
Just for the sake of interest, please tell me, dear experts.
Does he freeze at all? (-)
2002-02-04 16:08
What about
At absolute zero -273 Celsius, everything freezes, even gases.
2002-02-04 16:18
People are strong in spirit, ...
... who drive at this temperature. But, unfortunately, I never had a chance
Good luck,
Sergey
2002-02-04 16:56
Re: And how
However, not all. This is at least oxygen, and nitrogen has a much lower temperature.
Good luck on the road!
2002-02-04 21:52
Re: At what temperature does gasoline freeze?
Hey!
I don’t remember exactly, but it doesn’t freeze up to -60 (for example, in Yakutia).
But the mixture that is sold at gas stations with gasoline can be recognized conventionally. So in February 98 or 99 my car 21061 decided not to start after refueling at the Hermes-Moscow gas station. An autopsy showed that the candles were dry. An autopsy of the carb gave the result - frozen gasoline in the float chamber. And it was 12-13 degrees ...
Good luck,
Andrey
2002-02-04 16:12
Why immediately scold the gas station
maybe it is water (condensate) in your tank? But it needs to be removed periodically, at least by adding alcohol to the BB.
2002-02-04 16:20
So I periodically 1 time in 5-7Kkm and add and add additives.
I just didn’t refuel there anymore, and that’s it - it didn’t freeze anymore.
I have that 21061 and at -30 it started normally, though on normal gasoline.
Good luck,
Andrey
PS. And therefore I scold, tk. I know that they still use water for water. And the condensate in the filling tanks is not removed - the fault of the gas station.
2002-02-04 16:37
Re: Why immediately scold the gas station
If the entire contents of the float chamber are frozen, then it is definitely not water, because gasoline does not mix with water. And if the water turns to ice, then pure gasoline will not freeze. A solution of gasoline with something organic froze there ...
Best regards, VLA (21103).
2002-02-04 17:58
I did not find articles in Yandex, so I will say
Push.
Gasoline consists of many hydrocarbons, the term "freezes" does not apply to it, since crystallization is unlikely to occur at once for all fractions at the same time.
We can talk about the thickening of gasoline at low temperatures.
Remember bitumen - a petroleum product, an amorphous substance. Seemingly solid, but flows, even at zero degrees.
For gasoline, the picture will be similar, only at lower temperatures. Which ones - you need to look at the reference book.
Bon Voyage!
2002-02-04 18:00
-15
German froze during the Second World War.
So they abandoned their cars near Moscow.
V.M. (Retired Casanova)
The freezing point (or transition from a liquid state to a solid) for each liquid has its own and it depends on the properties of the liquid molecule, as well as on atmospheric pressure.

If we take pure alcohol (ethanol with the formula C 2 H 5 OH), then it can be frozen only at an extremely low temperature of -115 ° C. This temperature cannot be found in any, even the coldest region of the Earth. Therefore, alcohols (not only ethyl) are used as antifreeze and anti-icing fluids in aviation.
At what temperature does vodka freeze?
Vodka, being a mixture of alcohol and water in a ratio of 4 to 6, freezes at a temperature of -25 ° C to -29 ° C, depending on the quality of alcohol, because the alcohol that is used in the manufacture of vodka is not chemically pure alcohol and contains organic impurities. By the way, freezing vodka is a great way to check its quality: if it's only -20 on the street, and the vodka is already frozen, then you have been slipped a low-quality product.
At what temperature does gasoline freeze?
Gasoline used in modern cars, freezes at a temperature of about -60 ° C, which, although it sometimes happens in the far north and in Antarctica, is still extremely rare. The freezing point of gasoline is lowered with the help of special additives (additives).
Diesel fuel, that is, diesel fuel, is of three types: summer, winter and arctic. Summer diesel freezes at -5 ° C, winter diesel freezes at about -35 ° C. Arctic diesel fuel freezes at -50 ° C, making it suitable for working in the extreme north.
At what temperature do acids freeze?
Sulfuric acid (H 2 SO 4) freezes at -11 ° C, 10% hydrochloric acid freezes at -18 ° C, nitric acid (HNO 3) freezes at -42 ° C,
Today is September 1st, 2017. Do you know what holiday is today?
Tell At what temperature do alcohol, gasoline, vodka and other liquids freeze friends on social networks:
›Gasoline
Petrol- a combustible mixture of light hydrocarbons with a boiling point of 33 to 205 ° C (depending on impurities). Density is about 0.71 g / cm³. The calorific value is approximately 10,200 kcal / kg (46 MJ / kg, 32.7 MJ / liter). Freezing point -72 ° C if special additives are used.
Petrol- a product of oil refining, which is a fuel with low detonation characteristics. Up to 50% of gasoline is produced from crude oil. This figure includes natural gasoline, cracked gasoline, polymerization products, liquefied petroleum gases and all products used as industrial motor fuels.
Gasolines are intended for use in piston engines internal combustion with forced ignition (from a spark). Depending on the purpose, they are divided into automobile and aviation.
Despite the differences in the conditions of use, automobile and aviation gasolines are characterized mainly by general quality indicators that determine their physicochemical and operational properties.
Modern automobile and aviation gasolines must meet a number of requirements that ensure economical and reliable operation of the engine, and the requirements of operation: have good volatility, which makes it possible to obtain a homogeneous fuel-air mixture of optimal composition at any temperature; have a group hydrocarbon composition that ensures a stable, detonation-free combustion process at all engine operating modes; do not change its composition and properties during long-term storage and do not have a harmful effect on parts fuel system, reservoirs, industrial rubber goods, etc. In recent years, the ecological properties of fuel have come to the fore.
Gasoline composition
Gasoline is a mixture of hydrocarbons consisting mainly of the limit 25-61%, unsaturated 13-45%, naphthenic 9-71%, aromatic 4-16% hydrocarbons with a hydrocarbon molecule length from C 5 to C 10 and the number of carbon atoms from 4 -5 to 9-10 with an average molecular weight of about 100D. Also, the composition of gasoline can include impurities - sulfur-, nitrogen- and oxygen-containing compounds.
Gasoline is the lightest fraction of the liquid oil fractions. This fraction is obtained in a number of different processes of oil sublimation. Therefore, the ease and reliability of starting the engine, the completeness of combustion, the duration of warm-up, the throttle response of the car and the intensity of wear of engine parts depend on the fractional composition of gasolines. Fractional composition of gasoline is determined according to GOST 2177-99.
Light fractions of gasoline characterize the starting properties of the fuel - the lower the boiling point of the fuel, the better the starting properties. To start a cold engine, it is necessary that 10% of gasoline boils away at a temperature not exceeding 55 degrees (winter grade) and 70 degrees (summer) Celsius. Winter gasoline grades have a lighter (than summer) fractional composition. Light fractions are needed only for the period of starting and warming up the engine.
The main part of the fuel is called the working fraction. Its volatility depends on: the formation of a combustible mixture under different operating modes of the engine, the duration of warm-up (translation from idle move under load), throttle response (the ability to quickly transfer from one mode to another). The content of the working fraction must coincide with 50% of the distillate. The minimum temperature range from 90% to the end of boiling improves fuel quality and reduces its tendency to condensation, which increases efficiency and reduces wear of engine parts. The boiling point of 90% of the fuel is sometimes referred to as the dew point.
Gasoline properties
Gasolines - flammable colorless or slightly yellow (in the absence of special additives) liquids with a density of 700-780 kg / m3? Gasolines are highly volatile and have a flash point in the range of 20-40 degrees Celsius. The boiling point of gasoline is in the range from 30 to 200 C. The pour point is below minus 60 degrees. When gasoline burns, water and carbon dioxide are formed. At a concentration of vapors in the air of 70-120 g / m3, explosive mixtures are formed.
Motor gasolines, due to their physical and chemical characteristics, must have the following properties:
-Homogeneity of the mixture;
- Fuel density - at +20 ° С should be 690… 750 kg / m2;
-Small viscosity - with its increase, the flow of fuel through the jets becomes difficult, which leads to a depletion of the mixture. Viscosity is highly temperature dependent. When the temperature changes from +40 to -40 ° C, the consumption of gasoline through the jet changes by 20 ... 30%;
- Evaporation - the ability to change from a liquid to a gaseous state. Automobile gasolines must have such volatility as to ensure easy engine start-up (especially in winter), its rapid warming up, complete combustion of fuel, and the formation of vapor congestions in the fuel system is excluded;
-Pressure of saturated vapors - the higher the vapor pressure during the evaporation of fuel in a confined space, the more intense the process of their condensation. The standard limits the upper limit of vapor pressure in summer - up to 670 GPa and in winter - from 670 to 930 GPa. Gasolines with a higher pressure are prone to the formation of steam plugs, when they are used, the filling of the cylinders decreases and engine power is lost, losses from evaporation increase during storage in car tanks and in warehouses;
-Low-temperature properties - the ability of gasoline to withstand low temperatures;
-Combustion of gasoline. By "combustion" as applied to car engines understand the rapid reaction of the interaction of fuel hydrocarbons with atmospheric oxygen with the release of a significant amount of heat. The temperature of the vapors during combustion reaches 1500 ... 2400 ° C.
Additives
Additives are substances added (usually in amounts of 0.05-0.1%) to fuels, mineral and synthetic oils to improve their performance properties. Additives include antiknock agents, antioxidants, corrosion inhibitors, etc.
Improving the quality of gasoline
First of all, one should not confuse the quality and grade (according to the octane number) of gasoline: gasoline of lower grades (for example, A-76) is not necessarily of lower quality than high-octane (rather, the opposite), but is simply designed for different working conditions (First of all, do not confuse two completely different specifications gasoline: octane number and grade. It is also not more environmentally harmful (again, rather the opposite, since it contains fewer additives, some of which are quite toxic).
It is possible to improve the quality of motor gasoline through the following measures:
- the use of lead compounds, which are harmful to both the engine and service personnel;
-reduction of sulfur content in gasoline to 0.05%, and in the future to 0.003%;
-Reducing the content of aromatic hydrocarbons in gasoline up to 45%, and in the future - up to 35%;
-normalization of the concentration of actual tar in gasoline at the place of application at a level of no more than 5 mg per 100 cm³;
dividing gasolines by fractional composition and saturated vapor pressure into 8 classes, taking into account the season of vehicle operation and the ambient temperature characteristic of a particular climatic zone. The presence of classes allows the production of gasoline with properties that are optimal for real ambient temperatures, which ensures the operation of engines without the formation of vapor locks at air temperatures up to +60 ° С, and also guarantees high volatility of gasoline and easy engine start at temperatures below -35 ° С;
introduction of detergents that prevent contamination and resinification of parts of the fuel equipment.
Automobile gasolines
In Russia, motor gasolines are produced in accordance with GOST 2084-77, GOST R 51105-97 and GOST R 51866-2002, as well as TU 0251-001-12150839-2015 Gasoline AI 92.95 (Alternative).
Automobile gasolines are subdivided into summer and winter (winter gasolines contain more low-boiling hydrocarbons).
The main brands of motor gasolines GOST R 51105-97:
Normal-80 - with a research octane number of at least 80;
Regular-92 - with a research octane number of at least 92;
Premium-95 - with a research octane number of at least 95;
Super-98 - with a research octane rating of at least 98
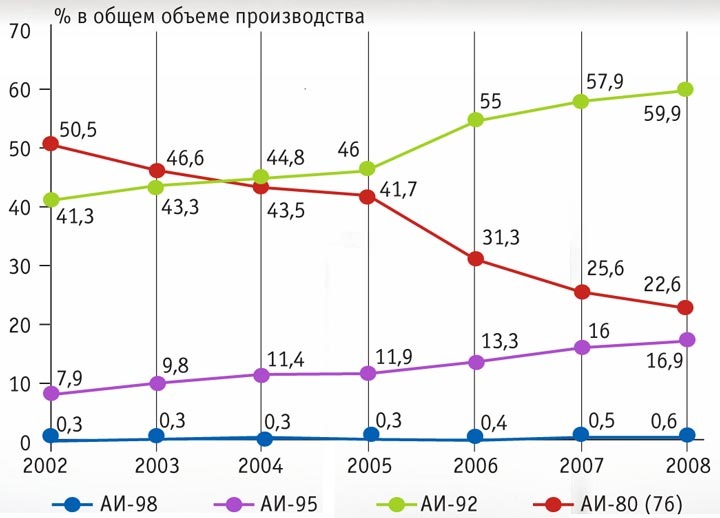
Production of gasoline of various grades
Automobile gasoline marking
In accordance with GOST R 54283-2010, motor gasolines are marked with three groups of characters separated by a hyphen (for example, "AI-92-4"):
- the letters "AI" (motor gasoline with an octane number, measured by the research method GOST 8226-82);
- octane number, measured by a research method (for example, 80, 92, 95 or 98);
-number 2, 3, 4 or 5 - gasoline class; the number coincides with the number of the environmental standard of the "Euro" series, which gasoline must comply with (2 for Euro-2, 3 for Euro-3, etc.).
Example. The AI-92-4 brand stands for motor gasoline with an octane rating of 92, measured by a research method, corresponding to the fourth environmental class (Euro-4 standard). Since the production of harmful leaded gasoline has been officially discontinued in Russia since 2003, all gasolines are considered unleaded, and this fact is not displayed in the marking in any way.
Raw materials for gasoline production
The raw material for the production of gasoline is oil. Oil is a natural liquid mixture of various hydrocarbons with a small amount of other organic compounds; a valuable mineral that often occurs together with gaseous hydrocarbons (associated gases, natural gas).
Crude oil compounds are complex substances consisting of five elements - C, H, S, O and N, and the content of these elements ranges from 82-87% carbon, 11-15% hydrogen, 0.01-6% sulfur, 0-2% oxygen and 0.01-3% nitrogen.
Hydrocarbons are the main components of oil and natural gas. The simplest of these, CH4 methane, is the main component of natural gas. All hydrocarbons can be subdivided into aliphatic (with an open molecular chain) and cyclic, and according to the degree of unsaturation of carbon bonds - into paraffins and cycloparaffins, olefins, acetylenes and aromatic hydrocarbons. Conventional well oil is a greenish brown, highly flammable oily liquid with a pungent odor.
Chemically, oils are very different and range from paraffinic oils, which are composed mostly of paraffinic hydrocarbons, to naphthenic or asphaltenic oils, which contain mainly cycloparaffinic hydrocarbons; there are many intermediate or mixed types. Compared to naphthenic or asphaltene oils, paraffinic oils usually contain more gasoline and less sulfur and are the main raw material for obtaining lubricating oils and paraffins. Naphthenic crude oils generally contain less gasoline but more sulfur and fuel oil and asphalt.
Gasoline production technology
Distillation
The incoming oil is heated in the coil to about 320 ° C, and the heated products are fed to intermediate levels in the distillation column. Such a column can have from 30 to 60 spaced trays and troughs, each of which has a bath of liquid. Rising vapors pass through this liquid, which are washed by condensate flowing down. With proper control of the backflow rate (i.e. the amount of distillates pumped back into the re-fractionation column), it is possible to produce gasoline at the top of the column, kerosene and light flammable distillates with well-defined boiling ranges at successively decreasing levels. Typically, in order to improve further separation, the distillation residue from the distillation column is subjected to vacuum distillation.
Thermal cracking
The tendency to further decompose the heavier fractions of crude oils when heated above a certain temperature has led to very important success in the use of the cracking process. When high-boiling oil fractions decompose, carbon and carbon bonds are broken, hydrogen is stripped away from hydrocarbon molecules and thus a wider range of products is obtained compared to the composition of the original crude oil. For example, distillates boiling in the temperature range of 290–400 ° C, as a result of cracking, give gases, gasoline and heavy tar-like residual products. The cracking process increases the yield of gasoline from crude oil by breaking down heavier distillates and residues from primary distillation.
Catalytic cracking
A catalyst is a substance that accelerates the course of chemical reactions without changing the essence of the reactions themselves. Many substances have catalytic properties, including metals, their oxides, and various salts.
Goodry process. E. Goodry's studies of refractory clays as catalysts led to the creation in 1936 of an effective catalyst based on aluminosilicates for the cracking process.
In this process, medium-boiling oil distillates were heated and transferred into a vaporous state; to increase the rate of cleavage reactions, i. e. cracking process, and changes in the nature of the reactions, these vapors were passed through the catalyst bed. The reactions took place at moderate temperatures of 430–480 ° C and atmospheric pressure, in contrast to thermal cracking processes, where high pressures... The Goodry process was the first catalytic cracking process to be successfully implemented on an industrial scale.
Reforming
Reforming is the process of converting linear and non-cyclic hydrocarbons into benzene-like aromatic molecules. Aromatic hydrocarbons have a higher octane number than other hydrocarbon molecules and are therefore preferred for the production of modern high-octane gasoline.
There are two main types of reforming - thermal and catalytic. In the first, the corresponding fraction of the primary distillation of oil is converted into high-octane gasoline only under the influence of high temperature; in the second, the transformation of the initial product occurs with the simultaneous action of both high temperature and catalysts. Older and less efficient thermal reformers are still in use, but in developed countries almost all thermal reformers have been replaced by catalytic reformers.
While gasoline is the preferred product, almost all of the reforming is carried out on platinum catalysts supported on an alumina or aluminosilicate support.
Reactions that increase octane during catalytic reforming include:
-dehydrogenation of naphthenes and their transformation into the corresponding aromatic compounds;
-transformation of linear paraffinic hydrocarbons into their branched isomers;
-hydrocracking of heavy paraffinic hydrocarbons into light high-octane fractions;
- the formation of aromatic hydrocarbons from heavy paraffinic ones by the elimination of hydrogen.
Polymerization
In addition to cracking and reforming, there are several other important processes for the production of gasoline. The first of these, which became economically viable on an industrial scale, was the polymerization process, which made it possible to obtain liquid gasoline fractions from olefins present in cracked gases.
Polymerization of propylene, an olefin containing three carbon atoms, and butylene, an olefin with four carbon atoms per molecule, gives a liquid product that boils in the same range as gasoline and has an octane number of 80 to 82. Refineries using polymerization processes usually operate on fractions of cracked gases containing olefins with three and four carbon atoms.
Alkylation
In this process, isobutane and gaseous olefins react under the action of catalysts and form liquid isoparaffins having an octane number close to that of isooctane. Instead of polymerizing isobutylene to isooctane and then hydrogenating it to isooctane, the process reacts isobutane with isobutylene to form isooctane directly.
All alkylation processes for the production of motor fuels are carried out using either sulfuric or hydrofluoric acid as catalysts at a temperature of 0–15 ° C first and then 20–40 ° C.
Isomerization
Another important way of obtaining high-octane feedstocks for addition to motor fuels is the isomerization process using aluminum chloride and other similar catalysts.
Isomerization is used to increase the octane number of natural gasoline and straight chain naphthenes. Improvement in antiknock properties occurs as a result of the conversion of normal pentane and hexane to isopentane and isohexane.
Isomerization processes are gaining importance, especially in countries where catalytic cracking to increase gasoline yields is carried out on a relatively small scale. With additional ethylation, i.e. the introduction of tetraethyl lead, the isomers have octane numbers from 94 to 107 (currently this method was abandoned due to the toxicity of the resulting volatile alkyl lead compounds that pollute the environment).
Hydrocracking
The pressures used in hydrocracking processes range from about 70 atm. for converting crude oil to liquefied petroleum gas (LP-gas) up to more than 175 atm., when complete coking and high yield conversion of vaporous oil to gasoline and jet fuel occurs. The processes are carried out with fixed beds (less often in a fluidized bed) of the catalyst. The fluidized bed process is used exclusively for oil residues - fuel oil, tar. Other processes also used residual fuels, but mainly high-boiling petroleum fractions, and in addition, low-boiling and middle distillate straight-run fractions. The catalysts in these processes are sulfided nickel-aluminum, cobalt-molybdenum-aluminum, tungsten materials and noble metals such as platinum and palladium on an aluminosilicate base.
Where hydrocracking is combined with catalytic cracking and coking, at least 75–80% of the feedstock is converted to gasoline and jet fuel. The production of gasoline and jet fuels can easily vary depending on seasonal demand. At high flow hydrogen product yield is 20-30% higher than the amount of raw materials fed into the unit. With some catalysts, the plant will run efficiently for two to three years without regeneration.
Gasoline classification
All gasolines differ from each other, both in composition and in properties, since they are obtained not only as a product of primary sublimation of oil, but also as a product of associated gas (gasoline) and heavy oil fractions (cracked gasoline).
Gasolines are classified for various reasons, including boiling ranges, octane numbers, sulfur content:
-Cracking gasolines
-Gas petrol
-Pyrolysis gasolines
-Leaded gasolines
-Cracking gasolines
Cracked gasolines contain a significant percentage of those components that, when mixed, form motor fuel. However, their direct use in many countries is legally limited, since they contain a noticeable amount of olefins, namely, olefins are one of the main reasons for the formation of photochemical smog.
Cracked gasoline is a product of additional oil refining. Conventional oil distillation yields only 10–20% of gasoline. To increase its amount, heavier or higher-boiling fractions are heated in order to break up large molecules to the size of molecules that make up gasoline. This is called cracking. The cracking of fuel oil is carried out at a temperature of 450–550 ° C. Thanks to cracking, up to 70% of gasoline can be obtained from oil.
Gas petrol
Gasoline is a product of associated petroleum gas processing, containing saturated hydrocarbons with at least three carbon atoms. Distinguish between stable (BGS) and unstable (BGN) variants of natural gasoline. BGS is of two brands - light (BL) and heavy (BT). It is used as a raw material in petrochemistry, in organic synthesis plants, as well as for compounding motor gasoline(obtaining gasoline with desired properties by mixing it with other gasolines).
Pyrolysis gasolines
Pyrolysis is cracking at temperatures of 700–800 ° C. Cracking and pyrolysis make it possible to bring the total gasoline yield up to 85%. It should be noted that the discoverer of cracking and the creator of the project for an industrial plant in 1891 was the Russian engineer V.G. Shukhov.


And what is really not happy is the prices. Prices in the country with the largest reserves of oil and gas, their largest exporting country. And the tendency for these prices to rise. From year to year.
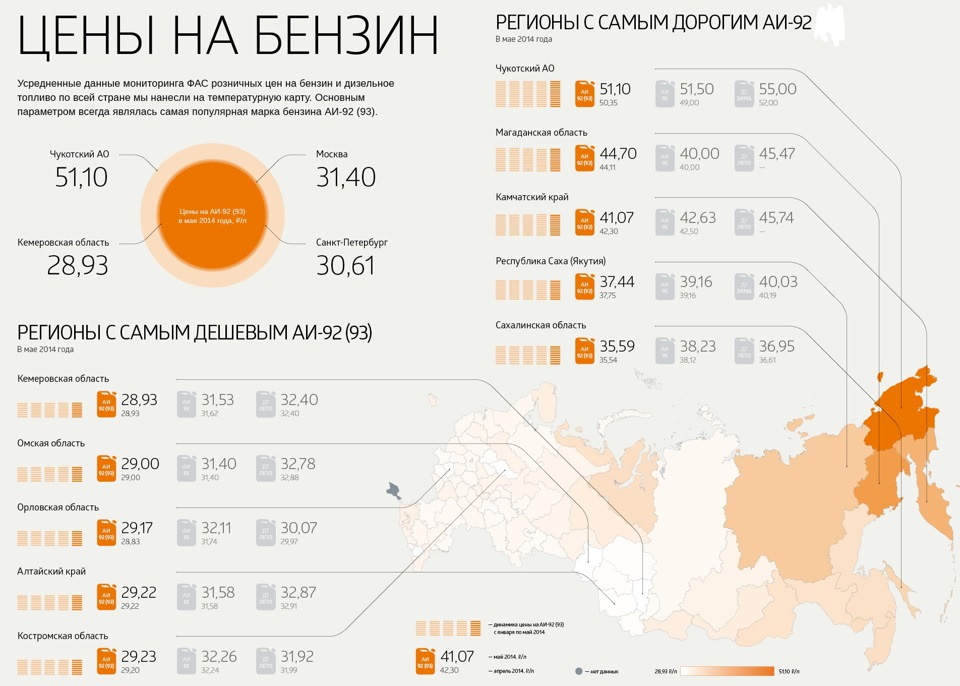
It is also upsetting that GOSTs are morally and practically outdated:
GOST 2084-77-introduced in 2002 (production standards)
GOST 2177-99-since 1999 (friction composition)
GOST R 51105-97 - since 1999 (gasoline grades)
GOST R51105-97 - since 1997 (production requirements for AI-95)
GOST 8226-82-since 1983, since 2002 amended (research method for determining RH)
but, of course, there are also fresh-2009 and 2015 introductions (or amendments)
We have such an army of officials and bureaucrats that we can allocate finances and people for updating and amending the main GOST standards, I think they can and should, but why? this is an additional cost
Well, for myself, I finally decided, only the 95th pour, especially after such an early engine bulkhead.
1 year
The question is fair especially for winter period time. To be honest, almost everything can freeze on our planet, gasoline is no exception, the whole question is - at what temperature? You know, they often write to me with such questions - help me, gasoline is frozen from the fuel pump to the engine - what to do? To be honest, this is not a completely correct understanding of the issue, the point is not in fuel, but in our "honest and valiant" gas stations. But about this a little later, let's first understand the physics of the issue ...
Oil - if you dig into the chemical formula of this type of "fossil" - it becomes clear that this liquid consists of two main components - carbon (about 85%) and hydrogen (about 15%), hence the name hydrocarbon! But there is simply NO definite formula! That is, it is different - it is combined in a large number of connections. Which we extract and get further from them fuel - gasoline, kerosene and diesel.
Gasoline is a volatile compound, it evaporates strongly and, unlike diesel, it is more toxic. It is produced in several ways - by direct distillation from oil or by catalytic and thermal cracking. Distillation is the "old way", because the resulting fuel is only about 10 - 15%, but cracking is already something new - if you do not go into complex technical processes, then this is the separation of molecules into components - the output is 4 - 5 times more fuel, and better quality.
Why gasoline as fuel?
Why not! This is a highly energetically valuable product, you know - that a liter of this fuel can produce 10,500 - 11,000 kilocalories of energy, just think about it! For example, this is enough - to lift a weight of 4000 tons to a height of one meter (taking into account the correct supply of energy). Therefore, a long time ago it was decided to build internal combustion engines on one of the types of hydrocarbon fuel. In fairness, all three types are now used: - kerosene, gasoline and diesel.

This is why it is so difficult to replace internal combustion engines with electric ones. In spite of high efficiency electric motor, it needs serious energy infusions from the batteries, and in our time, to my great regret, they are NOT PERFECT.
About temperature and freezing
The oil itself becomes thick already at - 25 - 30 degrees Celsius, but gasoline is it, if you want a volatile compound, it has much more low level freezing.

For the middle lane, where frosts are about - 20 - 35 degrees, you don't have to worry about anything, freezing of gasoline in the tank, in the cold, is simply physically impossible here.
There is still no exact information about freezing! There is a very high dependence of the fuel on additives in it, on the method of production and purification. However, there is such generally accepted information - popular brands like AI - 92, AI - 95 and AI - 98 have fairly low freezing thresholds: they start from - 72 degrees Celsius! If they can freeze, then only at the poles of our planet. According to other information, high-purity gasolines (according to the EURO 6 level) have a freezing threshold of - 118 degrees Celsius. At this temperature, gasoline does not become solid, it looks like liquid rubber or molten paraffin - a thick jelly-like substance.

To be honest, in the Arctic, there is a special gasoline, it is called “Arctic”. Its threshold is even lower, so with the help of a special formula and additives it remains liquid up to - 150 degrees Celsius, which is more than enough, yet there are practically NO such temperatures on earth!
But fuel freezing is not the worst thing - it needs to leave behind the possibility of ignition, otherwise it will be of little use, the so-called viscosity! So Russian GOSTs (GOST R 51105-97 and GOST R 51866-2002 - "with changes"), characterize not only the sulfur content in the fuel, but also the minimum temperature at which a flash should occur in the engine cylinders, now it is equal to 62 degrees. That is, at this temperature, gasoline of popular brands should ignite and not thicken.
The fuel supply is frozen, what should I do?
Now let's move on to our everyday life and questions from readers, why is gasoline not supplied to the injector or carburetor from the tank? What's frozen? Of course not! All the wine is here as fuel, sometimes by the owners themselves.
SO : I will not "find fault" with all the gas stations now, there are still more or less conscientious ones. However, on many, especially "without a name" gasoline, they mercilessly "badyazh" - sometimes with diesel, sometimes with water, but you never know what, there is even fuel oil and oil.
What's happening? In summer and in warm weather, water and other "badyaga" floats at the bottom of the tank, sometimes falls on the filter mesh, but it does not let them through. That way they stay "filtered" and your motor runs clean without problems.
But with the onset of frost, the situation changes dramatically. Water freezes even at 0 degrees, diesel, if it is not "winter", also begins to thicken, and other petrochemical products too. Thus, a dense film forms on the grids of your fuel filter, which does not let fuel through the highway, it seems like it works, but the fuel does not go! This happens all the time, no one is immune from this.

You just need to take off fuel filter and clean it, it is also advisable to clean the tank from "deposits" at the bottom. Then performance will be restored in 90% of cases. This malfunction manifests itself precisely in severe frosts - remember this!
I also pointed out that sometimes the drivers themselves are to blame - why? It's also simple - water can get into the tank when refueling the car in rainy or snowy weather, or when filling gasoline from a canister through a regular funnel, and who knows - what is in this canister at the bottom! So when pouring gasoline into the tank from a can, use a funnel with a mesh, there will be less problems in winter.

Now the video version, we are watching.


