The use of nuclear fuel in reactors for the production of thermal energy has a number of important features due to the physical properties and the nuclear nature of the processes. These features determine the specifics of nuclear energy, the nature of its technology, special operating conditions, economic performance and environmental impact. They also determine the main scientific, technical and engineering problems that must be solved with the widespread development of reliable, economical and safe nuclear technology.
The most important features of nuclear fuel, manifested in its energy use:
1. high calorific value, i.e. heat release per unit mass of separated nuclides;
2. the impossibility of complete "burning" (fission) of all fissile nuclides for a one-time stay of fuel in the reactor, since in the reactor core, it is always necessary to have a critical mass of fuel and it is possible to “burn” only that part of it that exceeds the critical mass;
3. the ability to have partial, under certain conditions, complete and even extended reproduction (conversion) of fissile nuclides, i.e. obtaining secondary nuclear fuel from reproducing nuclear materials (238 U and 232 Th);
4. “burning” of nuclear fuel in a reactor does not require an oxidizing agent and is not accompanied by continuous discharge of “combustion” products into the environment;
5. The fission process is simultaneously accompanied by the accumulation of radioactive short-lived and long-lived fission products, as well as decay products that retain a high level of radioactivity for a long time. Thus, the fuel irradiated in the reactor and spent in it has an extremely high radioactivity and, as a result, decay heat, which creates special difficulties in handling irradiated nuclear fuel;
6. chain reaction of fission of nuclear fuel is accompanied by the release of huge fluxes of neutrons. Under the influence of high-energy neutrons (E>0.1 MeV) in the irradiated structural materials of the reactor (fuel cladding, fuel assembly parts, in-reactor devices, vessel), as well as in the coolant and biological protection materials, in the gaseous atmosphere filling the space between the reactor and its biological protection, many chemically stable (non-radioactive) elements are converted into radioactive ones. There is a so-called induced activity.
The high heat generating capacity of nuclear fuel is due to the significant intranuclear energy released during each act of fission of a heavy uranium or plutonium atom. During the combustion of fossil fuels, chemical oxidative processes take place, accompanied by a relatively low energy release.
During the combustion (oxidation) of a carbon atom, in accordance with the reaction C + O 2 → CO 2, about 4 eV of energy is released for each act of interaction, while during the fission of the nucleus of the uranium atom 235 U + n → X 1 + X 2, about 200 MeV of energy per fission event. Such a highly concentrated release of energy per unit mass leads to huge thermal stresses. The temperature difference along the radius of the fuel element reaches several hundred degrees.
In addition, the core materials experience enormous dynamic and radiation loads due to the coolant flow and the powerful radiation effect on the fuel and structural materials of high-density ionizing radiation flows. In particular, the radiation action of fast neutrons causes significant radiation damage in the structural materials of the reactor (embrittlement, swelling, increased creep). Therefore, special requirements are imposed on the materials used in reactors. One of them is the highest degree of purity from impurities (the so-called nuclear-grade materials). Due to this, the cross section of interaction and absorption (which is important for maintaining a fission chain reaction) of neutrons by materials is minimal.
The level of requirements for the composition and properties of materials used in reactor construction turned out to be so high that it initiated the development of a number of new and advanced technologies for the production of special materials and semi-finished products, as well as special methods and means for controlling their quality. At present, a technology has been developed and mastered for the industrial production of such materials as beryllium, graphite of nuclear purity, heavy water, zirconium and niobium alloys, calcium metal, boron and heat-resistant stainless steels, boron enriched with the 10 V isotope, and rare earth elements.
The high caloric content causes a sharp reduction in both the mass and physical volumes of nuclear fuel required to produce a given amount of energy. Thus, the storage and transportation of the feedstock (chemical concentrate of natural uranium) and finished fuel require relatively low costs. The consequence of this is the independence of the location of nuclear power plants from the area of production and manufacture of nuclear fuel, which significantly affects the choice of an economically advantageous geographical location of productive forces. In this sense, one can speak of the universal nature of nuclear fuel. Its nuclear-physical properties are the same everywhere, and the economy of use practically does not depend on the distance to the consumer. The ability not to link the location of nuclear power plants with the place of production and manufacture of nuclear fuel makes it possible to economically optimally locate them throughout the country, bringing them as close as possible to consumers of electrical and thermal energy. Compared to fossil fuel power plants, nuclear power plants do not experience difficulties associated with seasonal climatic conditions for the delivery and supply of fuel. Nuclear materials extracted from the subsoil and undergoing processing can be stored for any number of years at very low cost, without requiring large and expensive storage facilities.
The need for repeated circulation of nuclear fuel in the fuel cycle and the impossibility of its complete combustion during a one-time stay in the reactor is due to the need to maintain a fission chain reaction. A self-sustaining chain reaction in the core is possible only if there is a critical mass of fissile material in it in a given configuration and under certain conditions for slowing down and absorbing neutrons. Therefore, in order to obtain thermal energy in the reactor, when operating at the design power for a given time, it is necessary to have some excess of fissile nuclides in the core in excess of the critical mass. This excess creates a reactivity margin of the reactor core, which is necessary to achieve the specified or calculated fuel burnup. Burnup of nuclear fuel in the reactor core is called the process of spending fissile nuclides, primary and secondary, as a result of fission during their interaction with neutrons. Burnup is usually determined by the amount of released thermal energy or the amount (mass) of separated nuclides per unit mass of fuel loaded into the reactor. Therefore, in order to burn a certain amount of uranium in a reactor, it is necessary to load it with fuel having a significantly larger mass than the critical one. In this case, after reaching the specified burnup depth, when the reactivity margin is exhausted, it is necessary to replace the spent fuel with fresh fuel in order to maintain the fission chain reaction. The requirement to constantly keep in the reactor core a large mass of nuclear fuel, designed for a long period of operation to ensure a given power output, causes significant one-time costs for paying for the first fuel load and subsequent batches prepared for refueling. This is one of the essential and fundamental differences between the conditions for the use of nuclear fuel in power plants in comparison with organic fuel.
However, the spent fuel removed from the core will contain significant amounts of fissile materials and fertile nuclides of significant value. This fuel, after chemical purification from fission products, can be returned to the fuel cycle for reuse. The amount of fissile nuclides in the spent fuel, which remains unused during its one-time stay in the reactor, depends on the type of reactor and on the type of fuel and can be up to 50% of those initially loaded. Naturally, such valuable "waste" must be used. For this purpose, special technical facilities and facilities are being created for the storage, transportation and chemical regeneration of spent fuel (SFA). Fissile materials extracted from SFAs can be returned and repeatedly circulated through reactors and fuel enterprises of the nuclear industry: radiochemical plants that ensure the regeneration (purification of fission products and impurities) of the fuel unloaded from the reactor and its return to the fuel cycle after the necessary additional enrichment with fissile nuclides; steel plants for the production of new fuel elements, in which regenerated fuel is added to fresh, not irradiated in reactors. Thus, a characteristic feature of fuel supply in the nuclear power industry is the technical possibility and the need to return to the cycle (recycle) the fissile and fertile isotopes of uranium and plutonium that were not used under the conditions of a single stay in the reactor. To ensure uninterrupted fuel supply, the necessary capacities of fuel cycle enterprises are being created. They can be considered as enterprises that satisfy the "own needs" of nuclear energy as an industry. The concept of development of nuclear power engineering based on nuclear fuel breeder reactors is based on the possibility of recycling uranium and plutonium. In addition, the recycling of uranium and plutonium significantly reduces the need for natural uranium and for uranium enrichment capacity for thermal neutron reactors, which currently dominate the developing nuclear power industry. As long as there is no reprocessing of spent fuel, there is no recycling of uranium and plutonium. This means that thermal reactors can only be powered by fresh fuel derived from mined and processed uranium, while the spent fuel will be stored.
Breeding of nuclear fuel takes place in almost any reactor designed for energy production, which, along with fissile materials, contains fertile raw materials (238 U and 232 Th). If we do not consider the hypothetical case of using super-enriched (~ 90%) uranium fuel for some special reactors, then in all nuclear reactors used in the energy sector, there will be a partial, and under certain conditions, complete and even expanded reproduction of nuclear fuel - plutonium isotopes, having the same high calorific value as 235 U. Plutonium can be separated from spent fuel in chemical processing plants in its pure form and used to make mixed uranium-plutonium fuel. The possibility of producing plutonium in any thermal neutron reactor makes it possible to qualify any nuclear power plant as a dual-purpose enterprise: generating not only thermal and electrical energy, but also producing a new nuclear fuel - plutonium. However, the role of plutonium is manifested not only in its accumulation in spent fuel. A significant part of the resulting fissile isotopes of plutonium undergoes fission in the reactor, improving the fuel balance and contributing to an increase in the burnup of the fuel loaded into the core. The most expedient, according to today's ideas, is the use of plutonium in fast neutron reactors, where it makes it possible to provide a gain in critical mass, and, consequently, in loading compared to 235 U by 20-30% and to obtain very high coefficients exceeding unity. reproduction. The use of plutonium in the fuel load of thermal neutron reactors, although it does not allow to obtain a significant gain in critical mass and such high breeding rates as in fast neutron reactors, however, creates a great effect, increasing nuclear fuel resources.
In nuclear energy, in addition to uranium, there are opportunities for the development of thorium fuel cycles. At the same time, the natural isotope 232 Th is used to produce 233 U, which is similar in its nuclear properties to 235 U. However, at present it is difficult to expect any significant use of the uranium-thorium cycle in nuclear power engineering. This is explained by the fact that 232 Th, like 238 U, is only a fertile, but not fissile material, and the thorium processing technology has a number of specific features and has not yet been mastered on an industrial scale. At the same time, there is no shortage of natural uranium yet. Moreover, there is a continuous accumulation in warehouses of waste uranium ready for use as a fertile material in breeder reactors.
The absence of the need for an oxidizer to generate energy is one of the key environmental benefits of using nuclear power compared to hydrocarbons. Gas emissions from nuclear power plants are mainly due to the needs of the station's ventilation systems. Unlike nuclear power plants, millions of cubic meters of combustion gases are released into the air every year. These include, first of all, oxides of carbon, nitrogen and sulfur, which destroy the ozone layer of the planet and create a great burden on the biosphere of adjacent territories.
Unfortunately, in addition to the advantages of nuclear energy, there are also disadvantages. These include, in particular, the formation of fission and activation products during the operation of a nuclear reactor. Such substances interfere with the operation of the reactor itself and are radioactive. However, the volume of generated radioactive waste is limited (much orders of magnitude less than waste from thermal plants). In addition, there are proven technologies for their purification, extraction, conditioning, safe storage and disposal. A number of radioactive isotopes extracted from spent fuel are actively used in industrial and other technologies. With the further development of SFA processing technologies, there are also prospects for the extraction of fission products from it - rare earth elements of great value.
Nuclear power plants generate 10.7% of the world's electricity generation annually. Along with thermal power plants and hydroelectric power plants, they are working to provide humanity with light and heat, allow the use of electrical appliances and make our lives more convenient and easier. It just so happened that today the words "nuclear power plant" are associated with global catastrophes and explosions. Ordinary inhabitants do not have the slightest idea about the operation of the nuclear power plant and its structure, but even the most unenlightened people have heard and are frightened by the incidents in Chernobyl and Fukushima.
What is a nuclear power plant? How do they work? How dangerous are nuclear power plants? Do not believe the rumors and myths, let's figure it out!
What is a nuclear power plant?
On July 16, 1945, energy was extracted from a uranium nucleus for the first time at a military test site in the United States. The most powerful explosion of the atomic bomb, which brought a huge number of human casualties, became the prototype of a modern and absolutely peaceful source of electricity.
For the first time, electricity was obtained using a nuclear reactor on December 20, 1951 in the state of Idaho in the USA. To test the operability, the generator was connected to 4 incandescent lamps, and unexpectedly for everyone, the lamps lit up. From that moment on, mankind began to use the energy of a nuclear reactor to generate electricity.
The world's first nuclear power plant was launched in Obninsk in the USSR in 1954. Its power was only 5 megawatts.
What is a nuclear power plant? A nuclear power plant is a nuclear installation that produces energy using a nuclear reactor. A nuclear reactor runs on nuclear fuel, most often uranium.
The principle of operation of a nuclear installation is based on the fission reaction of uranium neutrons, which, colliding with each other, are divided into new neutrons, which, in turn, also collide and are also divided. Such a reaction is called a chain reaction, and it underlies the nuclear power industry. This whole process produces heat, which heats the water to a terribly hot state (320 degrees Celsius). Then the water turns into steam, the steam rotates the turbine, it drives an electric generator, which generates electricity.
The construction of nuclear power plants is now being carried out at a fast pace. The main reason for the growth in the number of nuclear power plants in the world is the limited reserves of fossil fuels, simply put, gas and oil reserves are running out, they are needed for industrial and municipal needs, and uranium and plutonium, which are fuel for nuclear power plants, are needed little, its reserves are still quite enough .
What is a nuclear power plant? It's not just electricity and heat. Along with generating electricity, nuclear power plants are also used to desalinate water. For example, there is such a nuclear power plant in Kazakhstan.
What fuel is used in nuclear power plants
In practice, several substances capable of generating nuclear electricity can be used in nuclear power plants; modern nuclear power plant fuel is uranium, thorium and plutonium.
Thorium fuel is not currently used in nuclear power plants, because it is more difficult to convert it into fuel elements, in short fuel elements.
Fuel rods are metal tubes that are placed inside a nuclear reactor. Inside the fuel elements are radioactive substances. These tubes can be called storage facilities for nuclear fuel. The second reason for the rare use of thorium is its complex and expensive processing after use at nuclear power plants.
Plutonium fuel is also not used in the nuclear power industry, because. this substance has a very complex chemical composition, which has not yet been learned to use correctly.

uranium fuel
The main substance that generates energy at nuclear stations is uranium. Uranium is mined today in three ways: open pit mining, closed mines, and underground leaching, by drilling mines. The last way is especially interesting. To extract uranium by leaching, a solution of sulfuric acid is poured into underground wells, it is saturated with uranium and pumped back.
The largest uranium reserves in the world are in Australia, Kazakhstan, Russia and Canada. The richest deposits are in Canada, Zaire, France and the Czech Republic. In these countries, up to 22 kilograms of uranium raw materials are obtained from a ton of ore. For comparison, in Russia, a little more than one and a half kilograms of uranium is obtained from one ton of ore.
Uranium mining sites are non-radioactive. In its pure form, this substance is not very dangerous for humans; a much greater danger is the radioactive colorless gas radon, which is formed during the natural decay of uranium.
In the form of ore, uranium cannot be used in nuclear power plants; it cannot give any reactions. First, uranium raw materials are processed into powder - uranium oxide, and after that it becomes uranium fuel. The uranium powder is converted into metal "tablets" - it is pressed into small, neat cones, which are fired for a day at monstrously high temperatures of more than 1500 degrees Celsius. It is these uranium pellets that enter nuclear reactors, where they begin to interact with each other and, ultimately, give people electricity.
About 10 million uranium pellets work simultaneously in one nuclear reactor.
Of course, uranium pellets are not thrown into the reactor just like that. They are placed in metal tubes made of zirconium alloys - fuel elements, the tubes are interconnected in bundles and form fuel assemblies - fuel assemblies. It is fuel assemblies that can rightly be called nuclear power plant fuel.

NPP fuel processing
After about a year of use, uranium in nuclear reactors needs to be changed. Fuel cells are cooled for several years and sent for cutting and dissolution. As a result of chemical extraction, uranium and plutonium are separated, which are reused and used to make fresh nuclear fuel.
The decay products of uranium and plutonium are used to manufacture sources of ionizing radiation. They are used in medicine and industry.
Everything that remains after these manipulations is sent to a red-hot furnace and glass is brewed from the remains, which then remains stored in special storage facilities. Why glass? It will be very difficult to get the remains of radioactive elements from it, which can harm the environment.
Nuclear power plant news is a new way to dispose of radioactive waste that has recently appeared. So-called fast nuclear reactors or fast neutron reactors have been created, which operate on reprocessed nuclear fuel residues. According to scientists, the remains of nuclear fuel, which are now stored in storage facilities, are capable of providing fuel for fast neutron reactors for 200 years.
In addition, new fast reactors can operate on uranium fuel, which is made from 238 uranium, this substance is not used in conventional nuclear power plants, because. it is easier for today's nuclear power plants to process 235 and 233 uranium, of which there is not much left in nature. Thus, new reactors are an opportunity to use huge deposits of uranium 238, which no one has used before.

How is a nuclear power plant built?
What is a nuclear power plant? What is this jumble of gray buildings that most of us have only seen on TV? How durable and safe are these structures? What is the structure of a nuclear power plant? At the heart of any nuclear power plant is the reactor building, next to it is the engine room and the safety building.
NPP construction is carried out in accordance with regulations, regulations and safety requirements for facilities working with radioactive substances. A nuclear power plant is a full-fledged strategic object of the state. Therefore, the thickness of the laying of walls and reinforced concrete reinforcement structures in the reactor building is several times greater than that of standard structures. Thus, the premises of nuclear power plants can withstand an 8-magnitude earthquake, tornado, tsunami, tornadoes and an airplane crash.
The reactor building is crowned with a dome, which is protected by internal and external concrete walls. The inner concrete wall is covered with a steel sheet, which in the event of an accident should create a closed air space and not release radioactive substances into the air.
Each nuclear power plant has its own spent fuel pool. Uranium pellets that have already served their time are placed there. After the uranium fuel is pulled out of the reactor, it remains extremely radioactive, in order for the reactions inside the fuel elements to stop occurring, it must take from 3 to 10 years (depending on the reactor device in which the fuel was located). In the cooling pools, the uranium pellets cool down, and the reactions inside them cease to occur.
The technological scheme of a nuclear power plant, or more simply, the layout of a nuclear power plant, can be of several types, as well as the characteristics of a nuclear power plant and the thermal scheme of a nuclear power plant, it depends on the type of nuclear reactor that is used in the process of generating electricity.

floating nuclear power plant
We already know what a nuclear power plant is, but it occurred to Russian scientists to take a nuclear power plant and make it mobile. To date, the project is almost completed. They called this design a floating nuclear power plant. As planned, a floating nuclear power plant will be able to provide electricity to a city with a population of up to two hundred thousand people. Its main advantage is the ability to move by sea. The construction of a nuclear power plant capable of movement is currently being carried out only in Russia.
NPP news is the imminent launch of the world's first floating nuclear power plant, which is designed to provide energy to the port city of Pevek, located in the Chukotka Autonomous District of Russia. The first floating nuclear power plant is named "Akademik Lomonosov", a mini-nuclear power plant is being built in St. Petersburg and is planned to be launched in 2016-2019. The presentation of the afloat nuclear power plant took place in 2015, then the builders presented an almost finished design of the FAPP.
The floating nuclear power plant is designed to provide electricity to the most remote cities with access to the sea. The nuclear reactor "Academician Lomonosov" is not as powerful as that of land-based nuclear power plants, but has a lifespan of 40 years, which means that the inhabitants of small Pevek will not suffer from a lack of electricity for almost half a century.
A floating nuclear power plant can be used not only as a source of heat and electricity, but also for water desalination. According to calculations, it can produce from 40 to 240 cubic meters of fresh water per day.
The cost of the first block of a floating nuclear power plant was 16.5 billion rubles, as we see, the construction of nuclear power plants is not a cheap pleasure.
NPP safety
After the Chernobyl disaster in 1986 and the Fukushima accident in 2011, the words nuclear power plant cause fear and panic in people. In fact, modern nuclear power plants are equipped with the latest technology, special safety rules have been developed, and in general, nuclear power plant protection consists of 3 levels:
At the first level, the normal operation of the NPP should be ensured. The safety of a nuclear power plant largely depends on a properly selected place for the location of a nuclear power plant, a well-created project, and the fulfillment of all conditions during the construction of a building. Everything must comply with regulations, safety instructions and plans.
At the second level, it is important to prevent the transition of the normal operation of the NPP into an emergency situation. For this, there are special devices that control the temperature and pressure in the reactors, and report the slightest changes in readings.
If the first and second levels of protection did not work, the third is used - a direct response to an emergency. Sensors fix the accident and react to it themselves - the reactors are shut down, radiation sources are localized, the core is cooled, and the accident is reported.
Of course, a nuclear power plant requires special attention to the safety system, both at the construction stage and at the operation stage. Failure to comply with strict regulations can lead to very serious consequences, but today most of the responsibility for the safety of nuclear power plants falls on computer systems, and the human factor is almost completely excluded. Taking into account the high accuracy of modern machines, one can be sure of the safety of nuclear power plants.
Experts assure that it is impossible to receive a large dose of radioactive radiation in stably operating modern nuclear power plants or being close to them. Even nuclear power plant workers, who, by the way, daily measure the level of radiation received, are exposed to radiation no more than ordinary residents of large cities.

nuclear reactors
What is a nuclear power plant? This is primarily a working nuclear reactor. Inside it, the process of generating energy takes place. Fuel assemblies are placed in a nuclear reactor, in which uranium neutrons react with each other, where they transfer heat to water, and so on.
Inside a particular reactor building are the following facilities: a water source, a pump, a generator, a steam turbine, a condenser, deaerators, a purifier, a valve, a heat exchanger, the reactor itself and a pressure regulator.
Reactors are of several types, depending on which substance acts as a moderator and coolant in the device. It is most likely that a modern nuclear power plant will have thermal neutron reactors:
- water-water (with ordinary water as both a neutron moderator and a coolant);
- graphite-water (moderator - graphite, coolant - water);
- graphite-gas (moderator - graphite, coolant - gas);
- heavy water (moderator - heavy water, coolant - ordinary water).
NPP efficiency and NPP power
The overall efficiency of a nuclear power plant (efficiency) with a pressurized water reactor is about 33%, with a graphite-water reactor - about 40%, and a heavy water one - about 29%. The economic viability of a nuclear power plant depends on the efficiency of a nuclear reactor, the energy intensity of the reactor core, the annual installed capacity utilization factor, etc.
Nuclear power plant news is a promise by scientists to soon increase the efficiency of nuclear power plants by one and a half times, up to 50%. This will happen if fuel assemblies, or fuel assemblies, which are directly placed in a nuclear reactor, are made not from zirconium alloys, but from a composite. The problems of nuclear power plants today are that zirconium is not heat-resistant enough, it cannot withstand very high temperatures and pressures, and therefore the efficiency of nuclear power plants is low, while the composite can withstand temperatures above a thousand degrees Celsius.
Experiments on the use of a composite as a shell for uranium pellets are being conducted in the USA, France and Russia. Scientists are working to increase the strength of the material and its implementation in nuclear energy.
What is a nuclear power plant? Nuclear power plants are the world's electrical power. The total electrical capacity of nuclear power plants around the world is 392,082 MW. The characteristic of a nuclear power plant depends primarily on its power. The most powerful nuclear power plant in the world is located in France, the power of the Sivo nuclear power plant (each unit) is more than one and a half thousand MW (megawatts). The power of other nuclear power plants ranges from 12 MW in mini-nuclear power plants (Bilibino NPP, Russia) to 1382 MW (Flamanville nuclear power plant, France). At the construction stage are the Flamanville block with a capacity of 1650 MW, nuclear power plants of South Korea Sin-Kori with a nuclear power plant capacity of 1400 MW.
Nuclear power plant cost
Nuclear power plant, what is it? This is big money too. Today, people need any way to generate electricity. Water, thermal and nuclear power plants are being built everywhere in more or less developed countries. The construction of a nuclear power plant is not an easy process, it requires high costs and investments, most often financial resources are drawn from state budgets.
The cost of a nuclear power plant includes capital costs - the cost of preparing the area, construction, putting equipment into operation (the amount of capital costs is prohibitive, for example, one nuclear power plant steam generator costs more than 9 million dollars). In addition, nuclear power plants also require operating costs, which include the purchase of fuel, the cost of its disposal, and so on.
For many reasons, the official cost of a nuclear plant is only an estimate; today a nuclear plant would cost around 21-25 billion euros. Building one nuclear unit from scratch would cost about $8 million. On average, the payback period for one station is 28 years, the service life is 40 years. As you can see, nuclear power plants are quite an expensive pleasure, but, as we found out, they are incredibly necessary and useful for us.


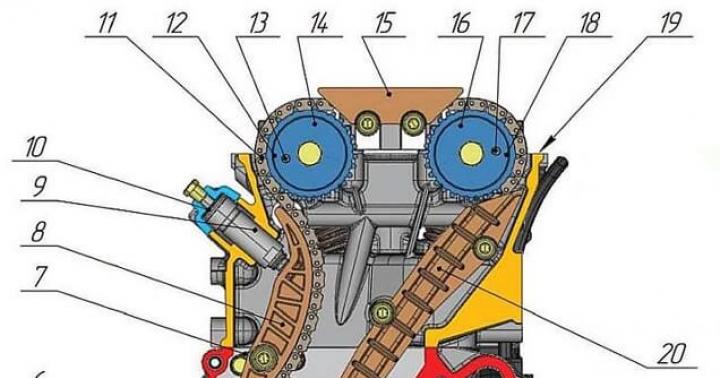
The central stage of the NFC is the use of nuclear fuel in a nuclear power plant reactor for the production of thermal energy. As an energy apparatus, a nuclear reactor is a generator of thermal energy of certain parameters, obtained by fission of uranium nuclei and plutonium, a secondary fuel element formed in the reactor (Fig. 6.22). The efficiency of converting thermal energy into electrical energy is determined by the perfection of the thermal-hydraulic and electrical circuits of nuclear power plants.
Features of nuclear fuel combustion in the reactor core, associated with the occurrence of various nuclear reactions with fuel elements, determine the specifics of nuclear energy, NPP operating conditions, economic indicators, environmental impact, social and economic consequences.
The efficiency of using nuclear fuel at NPPs with thermal neutron reactors is characterized by the average annual energy output per 1 ton (or 1 kg) of fuel loaded and spent in the reactor - the average depth of its burnup (its dimension is MW day / ton). In the process of uranium fuel burnout as a result of nuclear reactions, a significant change in its nuclide composition occurs. Figure 6.23 shows a typical graph of this process in relation to the design conditions of the VVER-1000 reactor core with initial enrichment x = 4.4% (44 kg / t) and the average design depth of fuel burnup В=40 10 3 MW day/t (or α =42 kg/t), and in Figure 6.24 - the calculated graph of the change in the nuclide composition of the fuel at x = 2% and В=20 10 3 MW day/t in the RBMK-1000 reactor core. It can be seen that as 235 U burns up, as a result of radiative capture of neutrons by 238 U nuclei, fissile isotopes of plutonium 239 Pu, 241 Pu and non-fissile isotopes 240 Pu, 242 Pu, and 236 U arise and accumulate. and the decay of other transuranium and transplutonium elements (Fig. 6.25), the number of which is relatively small and is not taken into account in economic calculations.

Figure 6.26 shows the dependence of the change in the nuclide composition in the uranium fuel of the PWR reactor, which has an initial enrichment of 3.44%, on the neutron fluence. The estimated contribution of fissile isotopes of plutonium (239 Pu and 241 Pu) to the total power output of the VVER-1000 nuclear reactor is more than 33%. This process also takes place in other thermal neutron reactors. The contribution of plutonium to fission and energy production is the greater, the higher the breeding ratio (BR) of plutonium and the greater the average fuel burnup.
The amount of accumulation of plutonium isotopes in spent fuel is essential for technical and economic calculations and assessments in the nuclear power industry. After being extracted from spent fuel during chemical processing, they are also commercial products of nuclear power plants.
The ratio of the mass z* of all or only fissile thermal neutrons z isotopes of plutonium accumulated in spent fuel to the mass α of fissile nuclei contained in 1 ton of spent fuel is commonly called the plutonium accumulation factor (KN):
КН=z/ α ; KH*=z*/α ,
where z* is the mass of all plutonium isotopes accumulated in the spent fuel (including the loss of 235U due to conversion to 236U without fission). For an approximate calculation of CV, one can use the graphs of changes in the nuclide composition of the fuel (see Fig. 6.23 and 6.24), built on the basis of nuclear-physical calculations. An increase in the average burnup depth B is accompanied (Table 6.13) by a decrease in the amount of plutonium in the spent fuel, but by an increase in its share in the total power output of the reactor. This proportion is the higher, the greater the value of the integral CV (the ratio of the number of formed fissile nuclides to the number of separated ones).
Table 6.13 Fuel burnup and plutonium accumulation in thermal reactors
|
fuel burnup, kg/t |
accumulation isotopes of plutonium, kg/t |
Coefficient accumulation of KH plutonium in spent fuel |
||||
|
fissile |
||||||
|
heavy water (CANDU type) |
||||||
|
high temperature gas-graphite |
||||||


When analyzing the material balance of 235 U in nuclear fuel, it is necessary to take into account its irreversible losses in the reactor core caused by the capture of neutrons by the 235 U isotope without fission 235 U+n → 236 U + γ .
A significant part of 235 U does not divide, but turns into an artificial non-fissile radioactive isotope 236 U. The probability of formation of 236 U from 235 U is equal to the ratio of the cross section for the radiative capture of a neutron by the 235 U isotope (σ n γ \u003d 98.36 for E n \u003d 0.0253 eV) to the sum of the cross sections for radiative capture and fission (σ ~ 580 barn). Thus, in the balance of the 235 U loaded into the reactor core, it is necessary to take into account not only the consumption of 235 U nuclei during its fission, but also the decrease (~ 15%) of the 235 U nuclei irreversibly lost to the formation of 236 U.
Figure 6.27 shows the level of accumulation of 236 U in a pressurized water reactor of a modern nuclear power plant with different initial fuel enrichment depending on its burnup depth.
In turn, the formation of 236 U leads to its consumption in the process of formation of new elements 237 Np and 238 Pu (see Fig. 6.22). The dependencies in Figure 6.27 take this process into account. At a burnup depth of 30 10 3 MW day/t, 0.35–0.40% 236 U is formed in thermal neutron reactors with fuel enrichment of ~ 3.4% 235 U.
With a content of 0.12% 236 U in the VVR core, the loss of the achievable burnup depth will be 10 3 MW day/t, at 0.4% 236 U - 2.5 10 3 MW day/t, at 1% 236 U – 5 10 3 MW day/t. In existing light water reactors, in order to compensate for the negative effect of 236 U and obtain the desired energy characteristics, it is necessary to increase the initial enrichment of 235 U fuel, which increases the cost of the nuclear fuel cycle.
The use of nuclear fuel in nuclear power plant reactors includes the following main operations:
- unloading, acceptance and storage at the FA warehouse of fresh fuel received from the supplier plant;
- assembly of fuel assemblies for loading into the reactor together with control rods;
- loading fuel assemblies into the reactor core (initial or in the order of periodic and partial refueling); efficient use of fuel in the reactor core (obtaining a given generation of thermal energy in the reactor).

The nuclear fuel spent in the reactor is reloaded into the spent fuel pool located in the reactor hall and stays there for several years. Such a long exposure makes it possible to significantly reduce the initial radioactivity and decay heat of fuel assemblies, to reject leaky assemblies and fuel rods in order to facilitate the task of transporting spent fuel from the territory of the NPP (Table 6.14).
From the spent fuel pools, the spent fuel is reloaded into transport containers installed on special railway platforms or on other vehicles. This operation ends at the nuclear power plant the longest - the central - stage of the nuclear fuel cycle. Some NPPs have long-term buffer storage for spent fuel or may store spent fuel assemblies in special casks adapted for long-term dry storage.
Fuel cycle types. There are a number of fuel cycles depending on the type of reactor being loaded and what happens to the spent fuel removed from the reactor. Figure 6.28 shows a diagram of an open (open) fuel cycle.
Spent fuel is stored for an indefinitely long period of time in the spent fuel pool at the nuclear power plant. In this regard, it is necessary to ensure the safety of handling, packaging and transfer of spent fuel to a permanent storage location using public storage facilities. In this cycle, the process of recovery or enrichment of fissile materials in the burnt fuel is not carried out. Figure 6.29 shows a cycle in which spent fuel is processed in such a way as to recover only uranium. Plutonium and transuranium elements are treated as high level waste (HLW) in this cycle.
The uranium is brought back to the enrichment plant in order to increase the percentage of enrichment from 0.8 to 3%, which is enough to reuse it as fuel for VVR. "Waste" requires proper handling, packaging and transport to a permanent storage location. A more complete fuel cycle is shown in Figure 6.30. Here, in addition to uranium, plutonium is also extracted. Because plutonium is a fissile material, it can be used as a fuel. Plutonium oxide mixed with uranium oxide can be reused in the WWR cycle. This fuel mixture, used in pilot assemblies in a number of commercial reactors, has demonstrated its successful use as a fuel for WWR.
Table 6.14 Change in specific activity and heat release in 1 ton of spent fuel unloaded from VVER at an average burnup of 33 10 3 MW day/t
|
Exposure, year |
Heat dissipation power, |
Activity, 104 |
However, plutonium recycle has not gained commercial acceptance due to a number of obstacles and limitations. Great interest in plutonium recycling was shown in Japan and Germany. In Japan, the main motive was to ensure the independence of obtaining fuel for nuclear power plants. In Germany, they wanted to take advantage of this to greatly simplify the disposal of high-level waste.



It is also possible to combine VVR and fast reactors based on the third version of the fuel cycle. Plutonium obtained from spent fuel can be used as the first fuel load of a fast reactor.
This is the most efficient use of plutonium, as its best qualities are found in the fast part of the neutron spectrum. This direction is used in France.
Plutonium produced in French refineries is stockpiled for later use in the fast reactor development program. A fast neutron reactor requires its own fuel cycle, with its own specifics and features. This specificity is due to the deep burnup of the fuel in the breeder (3 times or more greater than in VVR). Another cycle is based on the use of thorium, which, although not a fissile material, is converted into 23 U in the reactor. industrial development. The thorium cycle is used in high temperature gas reactors (in which the fuel is encapsulated in a matrix of graphite).
At present, in connection with the intensification of work to improve reactors and nuclear power plants as a whole, the positions of many countries regarding the choice of the type of nuclear fuel cycle are changing. More and more developers tend to choose a closed (closed) fuel cycle. On the other hand, one of the reports at the IAEA conference held in September 2004, which analyzed the situation with the choice of the type of NFC, taking into account the growing energy demand, states that the open, or single, fuel cycle has significant advantages compared to the closed cycle with respect to production costs, non-proliferation issues and the safety of fuel cycle operation. According to the report, there is enough natural uranium ore in the world to ensure the commissioning of 1,000 new reactors over the next fifty years. The “one-shot” method of using nuclear fuel will remain relatively cheap and safe until the uranium ore deposits are exhausted and the nuclear powers begin to process the accumulated spent nuclear fuel to produce plutonium, a non-natural, artificial by-product of burning uranium. At the same time, the situation with the cost of SNF and RW disposal operations is not analyzed. However, as uranium ore reserves are depleted, the cost of operating an open fuel cycle, the opposite of a closed cycle, may increase. Nevertheless, in order to avoid the incalculable risks associated with the use of a closed cycle, experts recommend that governments and leaders of the nuclear industry of nuclear powers continue to operate an open cycle in preference to a closed cycle due to the high cost of the SNF reprocessing process and developments in the field of new thermonuclear, or fast neutron, reactors. The authors of the report strongly advise directing fuel cycle research and development towards the development of technologies that would not, in a normal operation, i.e. a peaceful use of nuclear energy operation, lead to the production of weapons-usable materials, including uranium, fissile materials (such as plutonium) and small actinides. Closed fuel cycle practices currently implemented in Western Europe and Japan do not meet this criterion, the report says. Therefore, its authors say, fuel cycle analysis, research, development and testing should include a clear assessment of the possible risk of nuclear proliferation and the measures necessary to minimize this risk. If, however, the most likely forecast for the future of nuclear power is the global growth of the nuclear industry based on an open fuel cycle, then, the authors of the report say, international agreements on the storage of spent fuel should be put into effect within the next ten years, which should significantly reduce potential risk of nuclear proliferation.
In the future large-scale nuclear power industry on fast neutrons in the zone of nuclear reactions, not only the fission of actinides should be carried out, but also the production of plutonium isotopes, an excellent nuclear fuel, from the raw nuclear fuel uranium-238. With a breeding ratio above 1, more plutonium can be obtained in the discharged nuclear fuel than it burned down. The unloaded nuclear fuel from fast nuclear reactors must go to a radiochemical plant, where it will be removed from fission products that absorb neutrons. Then the fuel, consisting of uranium238 and actinides (Pu, Np, Cm, Am), sufficient to carry out a nuclear chain reaction, together with an additive of depleted uranium, is again loaded into the core of a nuclear power plant. In a fast neutron nuclear reactor, radiochemical processing can burn almost all of the uranium-238.
In the opinion of the authors of the report, fast-neutron nuclear reactors will prevail in large-scale nuclear power engineering. The fuel unloaded from these reactors contains a large amount of actinide isotopes (Pu, Np, Cm, Am), it is characterized by a large burnup depth, which means that there will be more fission products per unit mass of nuclear fuel.

There is still a need to create radiochemical technologies that provide:
- nuclear safety, taking into account a much larger number of small actinides with their own critical masses;
- deep purification of fission products from actinides, so as not to create difficulties in their storage, burial and transmutation;
- maximum reduction in the mass of technological waste;
- better purification of gases arising from radiochemical processing from iodine, tritium, krypton, radioactive aerosols;
- radiation safety of operating personnel;
- obtaining chemical elements needed by the national economy, for example, a pure α-source;
- the possibility of multiple use of materials located in the zone of nuclear reactions and consisting of valuable metals (Ni, Cr, Nb, Mo. Ti, W, V), which have acquired induced activity;
- economically viable radiochemical processing, competitive in comparison with the extraction of natural uranium for future energy.

Currently, spent nuclear fuel from four Russian nuclear power plants (Novo-Voronezh, Balakovo, Kalinin, Rostov), three Ukrainian (South-Ukrainian, Khmelnitsky, Rovno) and Kozloduy NPP (Bulgaria) is being stored in the plant's "wet" storage facility RT-2 for the regeneration of spent fuel on the territory of the Federal State Unitary Enterprise GCC, Zheleznogorsk (Russia). According to the project, the repository is designed for 6000 tons, it is supposed to be compacted with the possibility of placing 8600 tons of SNF. Irradiated fuel assemblies (SFAs) are stored under a water layer of at least 2.5 meters above the assembly, which ensures reliable protection of personnel from all types of radioactive exposure. After the spent nuclear fuel has been held in a wet storage facility, it will be placed in a dry SNF storage facility (KhOT-2) with a total capacity of 38,000 tons (of which 27,000 tons are for storing SFAs from RBMK-1000 reactors, 11,000 tons are for SFAs from VVER-1000 reactors), construction which is now in full swing at the plant and the first stage will be put into operation in December 2009. The XOT-2 storage complex will ensure safe long-term storage of spent nuclear fuel from the RBMK-1000 and VVER-1000 reactors and its further transfer for radiochemical processing or underground isolation. XOT-2 will be equipped with modern radiation and nuclear safety control systems.
Spent nuclear fuel is uranium that has worked in a nuclear reactor and contains radioactive fission products. Therefore, it is also called irradiated or burnt nuclear fuel.
How is SNF different from radioactive waste (RW)? First of all, the fact that SNF is a valuable product containing 2 useful components - unburned uranium and transuranium elements. In addition, fission products contain radionuclides (radioactive isotopes), which can be successfully used in industry, medicine, and also in scientific research.
After being removed from the reactor, spent nuclear fuel (SNF) retains radioactivity and releases heat. Therefore, for some time, such fuel is kept in pools under water to remove heat and protect against ionizing radiation. The next step could be:
- final disposal is the completion of an open fuel cycle as is done in the USA, Canada and Sweden.
- reprocessing of spent nuclear fuel for further use - a closed fuel cycle. The path of the closed fuel cycle was chosen by Russia, Great Britain, France and Japan.
Spent nuclear fuel is initially stored directly in the reactor building. Then it is moved to another location in special "dry storage" warehouses. In the closed fuel cycle for today's light water reactors, the fuel travels exactly the same path. Starting from uranium mines and factories, uranium goes through all the stages of transformation and enrichment for the manufacture of reactor fuel.After the fuel is removed from the reactor, the fuel rods are processed in refineries where they are crushed and dissolved in acid. After a special chemical treatment, two valuable products are recovered from the spent fuel: plutonium and unused uranium. Approximately 3% of the fuel remains as high-level waste. After bituminization, concreting or vitrification, these highly radioactive materials are subject to long-term disposal.

Spent nuclear fuel contains approximately 1% plutonium. This is a very good nuclear fuel that does not need any enrichment process. Plutonium can be mixed with depleted uranium to form mixed oxide or MOX fuel, which is supplied as fresh fuel assemblies for loading into reactors. It can be used to load into reactors. Recovered uranium can be returned for additional enrichment or supplied as fresh fuel for operating reactors. A closed fuel cycle is a more efficient system for maximizing the use of uranium without additional mining (in terms of energy units, the savings are about 30%). And although the industry immediately approved this approach, such schemes for the processing of spent nuclear fuel have not yet become widespread.
One of the reasons for such an incomplete use of the possibilities of uranium is that most of the existing industrial reactors belong to the so-called "light water" LWR reactors. They are good in many ways, but they are not designed to squeeze all the energy out of the fuel to the last watt. However, there are other types of reactors - the so-called "fast" (fast neutron reactors), capable of "processing" spent fuel to extract much more energy.
Why uranium?
Mankind has bound itself hand and foot with electric wires. Household appliances, industrial equipment, street lighting, trolleybuses, subways, electric trains - all these benefits of civilization are powered by the electrical network; they become meaningless "pieces of iron" if the current fails for some reason. However, people are already so accustomed to the constancy of the power supply that any shutdown causes dissatisfaction and even discomfort. And really, what should a person do if all the appliances went out at once, including the most beloved ones - a TV, a computer and a refrigerator? It is especially difficult to endure "separation" in the evening, when you so want after work or study, as they say, to extend daylight hours. Will a tablet save or a phone, but after all, they also have a charge that is not eternal. It is even worse to end up in a "prison cell", into which, at the behest of a blackout, an elevator cabin or a subway car can turn.
Why all this talk? And to the fact that "electrified" humanity needs stable and powerful sources of energy - first of all, electricity. With its shortage, blackouts will become annoyingly frequent, and the standard of living will decrease. To prevent an unpleasant scenario from becoming a reality, it is necessary to build more and more power plants: global energy consumption is growing, and existing power units are gradually aging.
But what can modern energy, which mainly burns coal and gas, offer to solve the problem? Of course, new gas installations that destroy valuable chemical raw materials, or coal blocks that smoke the sky. By the way, emissions from thermal power plants are a well-known environmental problem, but fossil fuel mining enterprises also cause harm to the environment. But its consumption is huge. For example, to ensure the operation of a conventional refrigerator during the year, it will be necessary to burn about a hundred kilograms of coal or hundreds of cubic meters of natural gas. And this is just one household appliance, of which there are many.
By the way, how much nuclear fuel will be needed for the said refrigerator to work for a whole year? It's hard to believe, but ... just one gram!
The colossal energy intensity of nuclear fuel made from enriched uranium makes it a worthy competitor to coal and gas. In fact, a nuclear power plant consumes a hundred thousand times less fuel than a thermal power plant. This means that mining for uranium mining is on a much smaller scale, which is important for the environment. Plus, there are no greenhouse and toxic gas emissions.
A power unit of a nuclear power plant with a capacity of one thousand megawatts will consume only three dozen tons of nuclear fuel per year, while a thermal plant of the same capacity will need about three million tons of coal or three billion cubic meters of gas. In other words, to obtain the same amount of electricity, you will need either several wagons with nuclear fuel per year, or several trains with coal ... per day.
What about renewable energy sources? They are, of course, good, but still need to be improved. Take, for example, the area occupied by the station. In the case of wind turbines and solar panels, it is two orders of magnitude higher than that of conventional power plants. For example, if a nuclear power plant (NPP) fits in an area of a couple of square kilometers, then a wind park or a solar field of the same capacity will occupy several hundred square kilometers. Simply put, the area ratio is like that of a small village and a very large city. In the desert, this indicator may not be important, but in the zone of agriculture or forestry - even how.
It should be mentioned that nuclear fuel is always ready to work, regardless of the season, day or weather vagaries, while the sun does not shine at night, and the wind blows when it pleases. Moreover, in some areas, renewable energy will not be profitable at all due to low solar energy flux or low average wind speed. For nuclear power plants, such problems simply do not exist.
These advantages of nuclear energy determined the outstanding role of uranium - as a nuclear fuel - for modern civilization.
Who got how much?
In one old Soviet cartoon, the animals solved an important task - they shared an orange. As a result, everyone, except for the wolf, was given a tasty juicy slice; the gray had to be content with the peel. In other words, he did not get a valuable resource. From this point of view, it is interesting to know how things are with uranium: do all countries of the world have its reserves, or are there deprived?
In fact, there is a lot of uranium on Earth, and this metal can be found almost everywhere: in the crust of our planet, in the oceans, even in the human body. The problem lies in its "dispersion", "smearing" over the earth's rocks, which results in a low concentration of uranium, most often insufficient for organizing economically profitable industrial production. However, in some places there are accumulations with a high content of uranium - deposits. They are unevenly distributed, respectively, and uranium reserves vary by country. Most of the deposits of this element "floated away" with Australia; in addition, Kazakhstan, Russia, Canada and the countries of South Africa were lucky. However, this picture is not frozen, the state of affairs is constantly changing due to the exploration of new deposits and the depletion of old ones.
Distribution of explored uranium reserves by country (for reserves with production costs< $130/кг)
A huge amount of uranium is dissolved in the waters of the World Ocean: over four billion tons. It would seem that the ideal "deposit" - I do not want to mine. Scientists have developed special sorbents for extracting uranium from sea water back in the 1980s. Why is this excellent method not universally applied? The problem is that the metal concentration is too low: only about three milligrams can be extracted from a ton of water! It is clear that such uranium will be too expensive. According to estimates, a kilogram will cost a couple of thousand dollars, which is much more expensive than the "land" counterpart. But scientists are not upset and invent more and more effective sorbents. So, perhaps in the near future this method of extraction will become competitive.
To date, the total number of explored uranium reserves with a production cost of less than $130 per kilogram exceeds 5.9 million tons. Is it a lot? Quite enough: if the total capacity of nuclear power plants remains at the current level, then uranium will last for a hundred years. By comparison, the proven reserves of oil and gas can be exhausted in just thirty to sixty years.
The top ten countries in terms of uranium reserves in their territory (for reserves with the cost of extraction< $130/кг)
However, we should not forget that, according to forecasts, the nuclear power industry will develop, so now it is worth thinking about how to expand its resource base.
One of the ways to solve the problem is to find and develop new deposits in a timely manner. Judging by the information available, this should not be a problem: only in the last few years have new deposits been found in some countries in Africa, South America, and also in Sweden. True, it is impossible to say with certainty how profitable the extraction of discovered reserves will be. It may happen that due to the low content of uranium in the ore and the difficulty of developing deposits, some of them will have to be left “for later”. The fact is that the prices for this metal are now quite low. From an economic point of view, there is nothing surprising. Firstly, there are still deposits of relatively easy to extract, and therefore cheap uranium in the world - it enters the market and “knocks down” the price. Secondly, after the Fukushima accident, some countries adjusted their plans for the construction of new nuclear power units, and Japan stopped all its nuclear power plants altogether - there was a drop in demand, further reducing the cost of uranium. But this is not for long. China and India have already entered the game, planning a large-scale construction of nuclear power plants on their territory. Other Asian countries, as well as African and South American countries, have less ambitious projects. Even Japan, apparently, will not be able to part with its nuclear power industry. Therefore, demand will gradually recover, and, coupled with the depletion of inexpensive deposits, this will lead to an increase in uranium prices. Analysts believe that the wait is not long, just a few years. Then it will be possible to think about the development of deposits left "for later".
It is interesting that the lists of countries with the largest reserves of uranium and those with the most developed nuclear power industry practically do not coincide. A third of the world's uranium "wealth" is in the bowels of Australia, but there is not a single nuclear power plant on the green continent. Kazakhstan, the world leader in the production of this metal, is just getting ready to build several nuclear power units. The countries of Africa, for economic and other reasons, are far from joining the world "nuclear" family. The only nuclear power plant on this continent is located in the Republic of South Africa, which recently announced its desire to further develop nuclear energy. However, so far even South Africa has taken a time out.
What remains to be done by the "atomic" giants - the USA, France, Japan - and China and India, advancing on their heels, if their needs are great, and the cat has wept for their own reserves? Of course, try to get control over deposits and uranium mining enterprises in other countries. This task is of a strategic nature, and, in solving it, the states enter into tough battles. Large companies are being bought up, political maneuvers are being undertaken, underground schemes are being implemented with bribery of the right people or judicial wars. In Africa, this struggle may even escalate - and is already escalating - into civil wars and revolutions, covertly supported by the leading states seeking to redistribute zones of influence.
In this regard, Russia is lucky: our nuclear power plants have their own quite decent uranium reserves, which are mined in the Trans-Baikal Territory, the Kurgan Region and the Republic of Buryatia. In addition, active exploration work is being organized. It is assumed that deposits in the Transbaikal region, Western Siberia, the Republic of Karelia, the Republic of Kalmykia and the Rostov region have great potential.
In addition, Rosatom also owns foreign assets - large blocks of shares in uranium mining enterprises in Kazakhstan, the USA, Australia, and is also working on promising projects in southern Africa. As a result, among the world's leading companies engaged in the production of uranium, Rosatom confidently holds the third place after Kazatomprom (Kazakhstan) and Cameco (Canada).
By studying the chemical composition of meteorites, some of which are of Martian origin, scientists have discovered uranium. True, its content turned out to be significantly lower than in terrestrial rocks. Yeah, now it’s clear why the Martians frequented us on their flying saucers.
But seriously, it is believed that uranium is present in all objects of the solar system. For example, in 2009 it was discovered in the lunar soil. Immediately, fantastic ideas arose, such as mining uranium on a satellite and then sending it to Earth. Another option is to "feed" the reactors of the lunar colonies, huddled close to the deposits. The deposits, however, have not yet been searched for; and from an economic point of view, such production still seems unrealizable. But in the future, who knows...
If you suffer for a long time, the fuel will turn out
The presence of uranium ore reserves is only one component of success. Unlike wood or coal, which do not require particularly complex preparation before entering the furnace, ore cannot simply be cut into pieces and thrown into the reactor. To explain why, it is necessary to mention a number of features inherent in uranium.
From a chemical point of view, this element is highly active, in other words, it tends to form various compounds; therefore, looking for its nuggets in nature, like gold, is a completely hopeless business. What then is called uranium ore? Rock containing very small amounts of uranium minerals. Often added: small, but enough for commercial production to be approved by economists. For example, today it is considered expedient to develop ore, a ton of which contains only a few kilograms or even hundreds of grams of uranium. The rest is empty, unnecessary rock, from which uranium minerals are to be isolated. But even they cannot yet be loaded into a nuclear reactor. The fact is that these minerals are most often oxides or insoluble salts of uranium in the company of other elements. Some of them may be of value to the industry, and the organization of their associated production can improve economic performance. But even if there is no such need, uranium must still be purified from impurities. Otherwise, nuclear fuel made from “dirty” uranium could cause reactor malfunctions or even an accident.
However, purified uranium also cannot be called nuclear fuel with complete certainty. The catch lies in its isotopic composition: for a thousand atoms of uranium in nature, there are only seven atoms of uranium-235, which is necessary for the fission chain reaction to occur. The rest is uranium-238, which practically does not fissile, and even absorbs neutrons. However, a natural uranium reactor is quite possible to start - provided that a very effective moderator is used, such as expensive heavy water or the purest graphite. Only they allow the neutrons formed during the fission of the uranium-235 nucleus to slow down so quickly in order to have time to get into other uranium-235 nuclei and cause their fission, and not be ingloriously captured by uranium-238. But for a number of reasons, the overwhelming majority of the world's reactors use a different approach: natural uranium is enriched in fissile isotopes. In other words, the content of uranium-235 atoms is artificially increased from seven to several dozen per thousand. Because of this, neutrons bump into them more often, and it becomes possible to use cheaper, albeit less effective, moderators, such as ordinary water.
Is enriched uranium already a final product? Again, no, since power reactors provide for the transfer of "nuclear" heat to a coolant that bathes the fuel - most often water. Due to the accumulation of fission products, the fuel - as it is in the operating reactor - becomes highly radioactive. Under no circumstances should it be allowed to dissolve in water. To do this, uranium is transferred to a chemically stable state, and is also isolated from the coolant, covering it with a metal shell. The result is a complex technical device containing enriched uranium compounds, which can be called nuclear fuel with full confidence.
The operations mentioned - uranium mining, its purification and enrichment, as well as the manufacture of nuclear fuel - are the initial stages of the so-called nuclear fuel cycle. It is necessary to get acquainted with each of them in more detail.
The half-life of uranium-238 is 4.5 billion years, while that of uranium-235 is only 700 million years. It turns out that the fissile isotope decays several times faster than the main one. If you think about it, this means that in the past the content of uranium-235 in the natural mixture of isotopes was greater than now. For example, one billion years ago, out of a thousand uranium atoms, sixteen had a nucleus with 235 nucleons, two billion years ago their number was thirty-seven, and three billion years before today - as many as eighty! In fact, the ore in those distant times contained uranium, which we today call enriched. And it could well happen that in some field a natural nuclear reactor would start working by itself!
Scientists believe that this is exactly what happened to several super-rich uranium deposits of the Oklo deposit, located on the territory of modern Gabon. 1.8 billion years ago, a nuclear chain reaction spontaneously started in them. It was initiated by neutrons produced during spontaneous fission, and then a high concentration of uranium-235 and the presence of water in the ore, a neutron moderator, worked. In a word, the reaction became self-sustaining and proceeded, now activating, now fading, for several hundred thousand years. Then the reactors went out, apparently due to a change in the water regime.
To date, it is the only known natural nuclear reactor. Moreover, at present, such processes cannot start in any field. The reason is quite understandable - there is too little uranium-235 left.
Try to dig
Uranium ores rarely come to the surface. Most often they lie at a depth of fifty meters to two kilometers.
Shallow deposits are developed by an open pit or, as it is also called, a quarry method. Hard rocks are drilled and blasted, and then, using loaders, they are placed in dump trucks and taken out of the quarry. Loose rocks are developed and loaded into mining trucks using conventional or rotary excavators, bulldozers are widely used. The power and size of this technique is amazing: for example, the already mentioned dump trucks have a carrying capacity of a hundred or more tons! Unfortunately, the scale of the quarry itself is also large, the depth of which can reach three hundred meters. After the completion of the work, it gapes like a huge hole in the earth's surface, and next to it, heaps of rock that covered the uranium deposits rise. In principle, a quarry can be covered with these dumps, planting grass and trees on top; but it will be prohibitively expensive. Therefore, the pits are gradually filled with water, and lakes are formed that are not subject to economic use due to the increased content of uranium in the water. There may also be problems associated with groundwater pollution, so uranium quarries require special attention.
However, the open mining of uranium is gradually becoming a thing of the past for a completely banal reason - deposits close to the surface are almost over. Now we have to deal with deeply hidden ores. Traditionally, they are developed by underground (mine) method. Just don't imagine stern bearded men with pickaxes crawling through the workings and chopping ore. Now the work of miners is largely mechanized. Holes are drilled in rock containing uranium - special deep holes into which explosives are placed. After the explosion, the crushed ore is taken with a bucket by a loading and hauling machine and runs along winding narrow galleries to the trolleys. The filled trolleys are carried to the vertical shaft of the mine by a small electric locomotive, and then with the help of a cage - a kind of elevator - the ore is raised to the surface.
Underground mining has a number of features. First, it can be beneficial only in the case of high-quality ores with a high uranium content, which occur no deeper than two kilometers. Otherwise, the costs of mining, mining and further processing of ore will make uranium practically “gold”. Secondly, the underground realm of uranium mines is a closed space in which radioactive dust and no less radioactive radon gas hovers. Therefore, miners cannot do without powerful ventilation and special protective equipment such as respirators.
In both open-pit and mine mining, ore is extracted in the form of rather large pieces. When scooping them up with the bucket of an excavator or a load-and-dump machine, the operator does not know whether he is selecting ore rich in uranium minerals, or waste rock, or something in between. After all, the deposit is not very homogeneous in its composition, and the use of powerful machines does not allow working finely and gracefully. But sending for further processing pieces that contain almost no uranium is at least unreasonable! Therefore, the ore is sorted using the main property of uranium, by which it is not difficult to detect it - radioactivity. Special ionizing radiation sensors make it possible, both during loading and already in the transport tank, to divide the ore into several grades according to the intensity of the radiation emitted by it. Waste rock is sent to dumps. Rich ore - to the hydrometallurgical plant. But ore with a small but noticeable amount of uranium is sorted again, more carefully. First, it is crushed, divided by size, after which the pieces are dumped onto a moving conveyor belt. An ionizing radiation sensor is installed above it, the signal from which enters the automated control system for shutters located at the end of the tape. The sensor is set up so that it reacts to a radioactive piece of ore passing under it containing uranium minerals. Then the damper turns and the ore falls into a special ore bunker, from where it is transported to the hydrometallurgical plant. In turn, the waste rock in no way "disturbs" the sensor and the damper, and falls into another box - into the dump.
Simplified scheme of radiometric sorting of ore (modern complexes are much more complicated)
The described scheme is approximate, fundamental: nothing prevents the sorting of ore at enterprises by other known methods. However, practice has shown that they are poorly suited for uranium ores. Therefore, radiometric sorting - with radiation detectors - gradually became the mainstream technology.
In reality, when sorting ore, a certain middle category is also distinguished, which, in terms of uranium content, cannot be attributed to either rich ore or waste rock. In other words, sending it to a hydrometallurgical plant is expensive (a waste of time and reagents), and it is a pity to send it to dumps. Such poor ore is piled in large piles and poured with sulfuric acid in the open air, gradually dissolving the uranium. The resulting solution is pumped for further processing.
At the hydrometallurgical plant, rich ore will have to be further crushed, almost to the state of dust, and then dissolved.
Ore is crushed in various mills - for example, drum-ball mills: crushed material and metal balls such as cannonballs are poured inside a rotating hollow drum. During rotation, the balls hit the pieces of ore, grinding them and grinding them into powder.
The crushed ore is “opened”, that is, partially dissolved by treating with sulfuric or nitric acid, or a mixture thereof. The result is a uranium solution containing many impurities. Sometimes, if the uranium ore contains a lot of natural carbonates, acid is not used. Otherwise, a reaction will occur that resembles extinguishing soda with vinegar - with intense release of carbon dioxide, and the reagent will be wasted. How to be? It turns out that such minerals can be "opened" with a soda solution. As a result, a solution of uranium will also be obtained, which will go for further processing.
But the remains of undissolved ore have to be sent to special tailings - not the most “friendly” objects in relation to the environment. It is worth recalling the waste rock separated during the sorting process: it is put into dumps. Both tailings and dumps contain small amounts of uranium, making them potentially hazardous. In this regard, the question arises: is it possible to organize mining in such a way as to cause minimal harm to nature and ensure the safety of workers?
It is possible, and it has been practiced for a long time. The extraction method in question is called downhole in-situ leaching. Its essence is that the deposit is “pierced” by many wells. Some of them, called pumping, are fed with sulfuric acid, which descends to the depth, passes through the ore and dissolves the uranium. Then the valuable metal solution is taken from the depths through other pumping wells.
What happens: no dumps, no tailings, no dust, no holes or unexpected sinkholes in the ground, but in the end - the same uranium solution? Yes. Moreover, the method of downhole underground leaching develops very poor ores, which are economically unprofitable to be mined by an open pit or mine method. But with such a set of advantages, there must be disadvantages! Well, firstly, drilling wells deeper than eight hundred meters is irrational from the point of view of costs. Secondly, the method does not work in dense, non-porous ores. Thirdly, sulfuric acid still disturbs the composition and behavior of groundwater in the deposit, although these disturbances “resolve” by themselves over time. It is much more dangerous if the solution spills over the surface or penetrates in a roundabout way - along cracks and faults - into groundwater. Therefore, the process is closely monitored by drilling control wells.
Borehole in-situ leaching
In order to avoid the mentioned problems, a “mine” version of underground leaching was invented: ore blocks in the workings are crushed by explosions, and then they are poured from above with a leaching solution (sulfuric acid), taking the uranium solution from below - through the drainage system.
In any case, today underground leaching is the most environmentally friendly way to extract uranium. This is one of the reasons for the explosive growth of its popularity. If in 2000 only fifteen percent of uranium was mined by underground leaching, then today this figure is almost fifty percent!
In-situ leaching becomes the leading uranium mining technology
Usually, uranium deposits are searched for using ionizing radiation sensors; more specifically, gamma radiation. First, an aircraft equipped with such sensors flies over the area. It is only in his power to fix the radiation anomaly - a slightly increased background over the field. Then a helicopter is launched into the business, which more slowly and more accurately “outlines” the boundaries of the promising area. In the end, prospectors with measuring instruments and drills come to this territory. Based on the results of their work, a map of the occurrence of uranium ores will be built and the cost of extraction will be calculated.
However, uranium ore deposits can signal themselves in other ways as well. For example, by changing the appearance of the plants growing above them: willow-herb petals, usually pink, turn white; blueberries turn green or turn white. The deep roots of the juniper growing above the deposit absorb uranium well, and it accumulates in branches and needles. Turning them into ash and checking for uranium content, one can understand whether it is worth extracting the main metal of nuclear energy in this area.
Cleanliness is the key to health (nuclear reactor)
The uranium solution obtained by "opening" the ore or in the process of underground leaching is not very pure. In other words, in addition to uranium, it contains a bunch of chemical elements found in the earth's crust: sodium and potassium, calcium and magnesium, iron, nickel and copper - and many others. Do not be surprised at the formation of such a thick "compote", because sulfuric acid is highly reactive and dissolves many natural substances; it's good that not all the ore is whole. But for the manufacture of nuclear fuel, the purest uranium is needed. If among the atoms of uranium there are atoms of impurities here and there, the reactor may not start or, even worse, break down. The causes of such problems will be discussed very soon, but for now, you can set the task: to purify uranium. And it is also desirable to get it in a solid form, convenient for transportation. Indeed, solutions are not suitable for transportation: they “like” to spill or seep through leaks too much.
In industry, this problem is solved in several ways. First, the solution is concentrated by passing through special materials that collect uranium on themselves - sorbents. The first opportunity for purification appears: the sorbents are selected in such a way that other elements almost do not “sit down” on them, remain in solution. Then the uranium is washed off the sorbent, for example, with the same sulfuric acid. This procedure may seem meaningless, if you do not explain that much less acid is needed for the "flush" compared to the volume of the original solution. This is how they kill two birds with one stone: they increase the concentration of uranium and partially remove unnecessary impurities.
The second purification stage is associated with the production of solid uranium compounds. They are precipitated from a concentrated solution by adding well-known "medical" reagents: ammonia, hydrogen peroxide, as well as alkalis or carbonates. It should be noted that uranium does not precipitate as a metal; it is generally not easy to obtain in a metallic form due to its high chemical activity - this has already been mentioned. Under the action of the mentioned regents, various sparingly soluble uranium compounds sink to the bottom of the apparatus. Dried and crushed, they are a yellow powder, which, due to its apparent resemblance to a cake, is often called "yellow cake". After calcining it at a high temperature, a less beautiful mixture of uranium oxides is obtained - a dirty green or even black color.
Yellow cake can be sent to uranium enrichment enterprises
Yellow cake or a mixture of uranium oxides is practically safe from a radiation point of view. Therefore, for transportation, they are loaded into 200-liter metal barrels or special containers. Being at a distance of one meter from such a container is not half as “harmful” as flying in an airplane, being exposed to cosmic radiation. But most people are not afraid to fly! So, there is no reason to be afraid of barrels with yellow cake.
When precipitating uranium compounds, they try to conduct the process in such a way that most of the impurities remain in solution. But some of them still manage to "break through". It is especially bad if elements that strongly absorb neutrons - boron, cadmium, rare earth metals - get into the product. Even in microconcentrations, they are able to interfere with the chain reaction of fission. Having made fuel from contaminated uranium, it will be possible to wonder for a long time why the reactor does not want to work normally.
In addition, undesirable impurities include elements that reduce the plasticity of nuclear fuel and cause it to swell and expand with increasing temperature. These include the naturally occurring silicon and phosphorus, as well as tungsten and molybdenum. By the way, plasticity is usually called the ability of a material to change its shape and size without collapsing. This is very important for fuel, which heats itself from the inside due to the nuclear chain reaction taking place in it, and, therefore, experiences temperature deformations. The high temperature should not lead to excessive expansion of the uranium fuel, otherwise it will break the containment and come into contact with the coolant. The consequence of such "communication" can be the dissolution of radioactive uranium fission products in a hot coolant (most often water) with their subsequent spreading through all pipelines and apparatuses. Probably, it is not necessary to explain that this threatens to worsen the radiation situation at the power unit: the doses received by the operating personnel will increase significantly.
As the saying goes, it's better to be overdressed than underdressed. Therefore, a third - final - stage of purification, called refining, is also required. Uranium compounds delivered in barrels or containers are dissolved in acid, now in nitric acid. The resulting solution is brought into contact with an extractant - a liquid organic substance that absorbs uranium, but not impurities. So, undesirable elements remain in solution, and uranium goes into the "organic". As a result of a series of subsequent operations, it is again brought into the form of oxides that already have the required "reactor" purity.
Now everything is fine, and you can proceed to the next stage - an artificial increase in the concentration of uranium-235.
Secrets of enrichment
It was already mentioned at the beginning of the chapter that in a natural mixture of uranium isotopes there is very little fissile uranium-235 and too much "lazy" uranium-238: for seven atoms of the first there are about nine hundred ninety-three atoms of the second. For most reactors currently operating, this is not suitable. They need fuel in which, out of a thousand uranium atoms, several dozen pieces belong to the isotope-235, and not just a few, as in natural uranium. And to create a bomb, almost pure uranium-235 is absolutely necessary.
Solving the problem of uranium enrichment, that is, increasing the content of a fissile isotope, is very difficult. It would seem, how so? After all, chemistry has a wide range of techniques for isolating substances from mixtures. It is possible to "pick out" only a few hundred grams of uranium from a ton of ore! Is it really impossible to do the same with isotopes: somehow separate one from the other? The problem is that the chemical properties of all isotopes of a certain element are the same, because they are determined by the number of electrons, not the composition of the nucleus. In other words, it is impossible to carry out such a reaction in which uranium-235, for example, would remain in solution, and uranium-238 would precipitate. With any manipulation, they both behave in a similar way. In the same way, it will not be possible to chemically separate the isotopes of carbon or potassium - in general, any element.
There is such a parameter - the degree of enrichment, which is the percentage (in percent) of uranium-235 in the total mass of uranium. For example, the degree of enrichment of natural uranium, in which there are seven fissile atoms for every thousand atoms, is 0.7%. In the case of nuclear fuel from nuclear power plants, this figure has to be raised to 3-5%, and for the production of the filling of an atomic bomb - up to 90% and higher.
How to be? It is necessary to find such properties in which the isotopes - at least minimally - would differ from each other. The first thing that comes to mind is the mass of an atom. Indeed, there are three more neutrons in the uranium-238 nucleus than in uranium-235; so the "lazy" isotope weighs a little more. And since mass is a measure of inertia, and it manifests itself in motion, the main ways of enriching uranium are associated with differences in the movement of its isotopes under specially created conditions.
Historically, the first enrichment technology was electromagnetic isotope separation. From the name it is clear that electric and magnetic fields are somehow involved in the process. Indeed, in this method, previously obtained uranium ions are dispersed by an electric field and launched into a magnetic field. Since the ions have a charge, in a magnetic field they begin to "carry", twist in an arc of a certain radius. For example, we can recall the division of uranium rays in a magnetic field into three streams - an effect discovered by Rutherford. Alpha and beta particles, which have an electric charge, deviate from a straight path, but gamma radiation does not. In this case, the radius of the arc along which a charged particle moves in a magnetic field depends on its mass: the more it weighs, the slower it turns. This can be compared to trying to fit into a sharp turn of two reckless drivers, one of whom is driving a car, and the other is a truck. It is clear that it is much easier for a passenger car to maneuver, while a truck may well skid. Something similar happens in a magnetic field with fast moving uranium-235 and uranium-238 ions. The latter are slightly heavier, have more inertia, and their turning radius is slightly larger: due to this, the stream of uranium ions is divided into two. Figuratively speaking, you can put two boxes, in one of which to collect the fissile isotope, uranium-235, and in the second - "unnecessary" uranium-238.
In a magnetic field, the trajectory of charged particles is curved, and the stronger, the lighter the particle
The principle of the electromagnetic isotope separation method: lighter uranium-235 ions move in a magnetic field along a trajectory of a smaller radius compared to uranium-238 ions
The electromagnetic separation method is good in almost all respects, except for productivity, which, as usual, limits its industrial application. Actually, that is why the American plant Y-12 in Oak Ridge, which produced enriched uranium for the bomb "Kid" dropped on Hiroshima using electromagnetic separation technology, closed back in 1946. It should be clarified that the Y-12 brought to a high degree of enrichment uranium, previously enriched in other, more productive ways. Their improvement just drove the last nail into the coffin of electromagnetic isotope separation technology - it is no longer used in industry.
Interestingly, electromagnetic separation is a universal method that allows you to isolate small amounts of any isotopes in pure form. Therefore, our analogue of Y-12 - plant 418, now known as the Elektrokhimpribor Plant (Lesnoy, Sverdlovsk Region), has technologies for producing more than two hundred isotopes of forty-seven chemical elements from lithium to lead. These are not just impressive numbers - the products of the plant are really needed by scientists, doctors, industrialists ... By the way, they are produced at the SU-20 facility, the same one that produced weapons-grade uranium with an enrichment level close to 90% in the early 1950s.
The first post-war decades became a time of active accumulation of arsenals of nuclear weapons. The solution of this problem had the highest priority, therefore, they did not particularly consider the costs - it was important to launch the mass enrichment of uranium. Emphasis was placed on gaseous diffusion, an extremely energy-intensive, but at the same time productive enrichment technology. Its roots lie in the field of gas theory, which states that at a certain temperature, the average speed of a gas molecule is inversely proportional to its mass: the heavier it is, the slower it moves. This difference is especially noticeable when moving along thin "tubes", the diameter of which is comparable to the size of the molecule. A clear, although not exact, example is the launch of paper boats in a stream: a small boat, carried away by a stream of water, will move quickly; but if you fold a large vessel of paper the size of a stream bed, then it will go more slowly, constantly touching the banks. Returning to uranium, we can say that the target isotope with 235 nucleons in the nucleus will move along the “tube” faster than uranium-238. At the exit from it, a gas enriched with a fissile isotope will be obtained. The only question is how to turn uranium into gas and where to get such a thin "tube".
"Gasification" of uranium is a mandatory requirement for technology based on the theory of gases. You can't write anything here. But after all, all uranium compounds are solids, which are difficult to melt, let alone evaporate. Although, if you think about it, there is one very successful compound - uranium hexafluoride, in which uranium is surrounded by six fluorine atoms. It readily turns into a gas already at 56 ° C, and bypassing the liquid state. In physics, such a process is usually called sublimation or sublimation. This phenomenon has long been known, and there is nothing surprising in it. Sublimation, for example, is used by village housewives who dry clothes in the cold - ice evaporates in dry air, simply passing the liquid state.
So you can imagine the uranium hexafluoride molecule
It turns out that uranium hexafluoride is very convenient from a technological point of view. At ordinary temperatures, it is solid and can be transported in special containers. It turns into a gas at a low temperature. Well, under a certain pressure, heated hexafluoride becomes a liquid that can be pumped through pipelines.
Another fortunate circumstance is that natural fluorine consists of only one isotope - fluorine-19. This means that the difference between the masses of the molecules of uranium-235 hexafluoride and uranium-238 hexafluoride is determined exclusively by uranium isotopes. Otherwise, separation would be too difficult or even impossible, since fluorine would have an excessive effect on the mass of the molecules.
The production of uranium hexafluoride in Russia is carried out by conversion - fluorination of various uranium compounds, for example, yellow cake or a mixture of oxides received from uranium mining enterprises. Molecular fluorine for these purposes is obtained from the natural mineral fluorite. It is treated with sulfuric acid to form hydrofluoric (hydrofluoric) acid, the electrolysis of which gives fluorine.
Interestingly, fluorination is simultaneously the fourth stage of uranium purification, since the fluorides of most harmful impurities are not highly volatile: uranium in the form of hexafluoride "flies away" from them into the gas phase.
Uranium hexafluoride has one big drawback: it is an aggressive and toxic substance. First, when it comes into contact with water or moisture in the air, poisonous hydrofluoric acid is released. Secondly, uranium itself is a general cellular poison that affects all organs. (Interestingly, its toxicity is chemical in nature, and practically unrelated to radioactivity). Therefore, uranium hexafluoride, which combines two hazards at once, should be transported and stored in special metal containers and under vigilant supervision. This ensures the safety of the population and the environment.
So, there is gas; But what about thin tubes? A suitable solution turned out to be porous partitions - plates pierced by many very small pores. The diameter of the latter must be of the order of ten nanometers, so that the molecules pass through them almost one by one. The need to manufacture partitions with pores of such a small size caused certain difficulties, but nevertheless the problem was solved using special approaches - nickel sintering or selective dissolution of one of the metals that make up the bimetallic alloy.
If we make a box with such a porous partition and pump uranium hexafluoride into it, molecules with a light isotope will pass through the partition a little faster. In other words, after it, uranium hexafluoride will be slightly enriched in the fissile isotope. If you send gas to the next same box, the degree of enrichment will become greater, and so on. True, to obtain a high degree of enrichment, cascades of thousands (!) of boxes installed one after another, called steps, are needed. But how to make uranium go up these steps? Only by pumping it with many compressors. Hence the disadvantages of the method: huge energy costs, the need to build millions of square meters of production space - the length of the workshop can reach one kilometer - and the use of expensive materials. True, all this is covered by a really high performance. That is why the gaseous diffusion enrichment technology has long remained the main one for such nuclear giants as the USA, France and China, which later joined them. Only in recent years have they begun an active transition to more economical gas centrifugation technology.
Scheme of operation of the gas diffusion stage
In the 1960s, the Angarsk electrolysis chemical plant (Irkutsk region, Russia), which was engaged in uranium enrichment using gas diffusion technology, consumed about one percent (!) of all electricity produced in the Soviet Union. Energy was supplied to it by the Bratsk and Irkutsk hydroelectric power stations. In fact, it was the largest consumer of electricity in the USSR.
In general, the first experience showed that gas diffusion can solve the problem, but at too high a price. The Soviet Union, drawn into the arms race, needed a more productive and less energy-consuming technology for uranium enrichment. It was not so easy for a war-weakened state to keep up with the United States with its powerful economic and energy potential. This was due, among other things, to the lack of electricity generation capacity in the European part of the country: that is why the enrichment plants were built in Siberia, where they could be powered by large hydroelectric power plants. But still, gaseous diffusion plants consumed too much energy, not allowing to increase the production of enriched uranium. Therefore, the USSR had to become a pioneer in the industrial application of an alternative technology - gas centrifuge.
Gas centrifugation consists in spinning a drum filled with gaseous uranium hexafluoride at high speed. Under the action of centrifugal force, the heavier uranium-238 hexafluoride is “squeezed out” to the drum wall, and uranium-235 hexafluoride, a lighter compound, remains near its axis. Using special tubes, you can pick up slightly enriched uranium from the center of the drum, and slightly depleted uranium from the periphery.
Scheme of operation of a gas centrifuge
From a technical point of view, the drum just discussed is the rotating part (rotor) of a gas centrifuge. It spins non-stop in an evacuated casing and rests with a needle on a thrust bearing made of a very durable material - corundum. The choice of material is not surprising, since the rotor speed can exceed 1500 revolutions per second - a hundred times faster than the drum of a washing machine. A fragile substance will not withstand such an impact. Additionally, so that the thrust bearing does not wear out and does not collapse, the rotor is suspended in a magnetic field so that it barely presses on the corundum with its needle. This technique, as well as the high precision of manufacturing parts of the centrifuge, allows it to rotate quickly, but almost silently.
As in the case of gaseous diffusion, one centrifuge is not a warrior in the field. In order to achieve the required degree of enrichment and productivity, they are combined into huge cascades consisting of tens of thousands (!) of machines. Simplified, each centrifuge is connected to two of its "neighbors". Uranium hexafluoride with a reduced content of uranium-235, taken from the wall in the upper part of the rotor, is sent to the previous centrifuge; and the gas slightly enriched in uranium-235, which is taken from the axis of rotation at the bottom of the rotor, goes to the next machine. Thus, more and more enriched uranium is supplied to each subsequent stage until a product of the required quality is obtained.
Receding into the distance cascades of gas centrifuges
Today, centrifuge separation is the main method for uranium enrichment, since this technology requires about fifty times less electricity than gas diffusion. In addition, centrifuges are less bulky than diffusion machines, making it easier to scale up production. The centrifugation method is used in Russia, Great Britain, Germany, the Netherlands, Japan, China, India, Pakistan, Iran; the transition to gas centrifuge technology in France and the USA is almost completed. In other words, there is no room left for gaseous diffusion.
Thanks to a long history of use and improvement, Russian gas centrifuges are the best in the world. For half a century, nine generations of high-speed cars have already changed, which gradually became more powerful and reliable. Thanks to this, the USSR successfully withstood the "nuclear race" with the United States, and when the most important task was completed, free capacities appeared. As a result, our country has become a world leader not only in the development and production of gas centrifuges, but also in the provision of commercial services for uranium enrichment.
Our gas centrifuges:
Traditionally, they have a height of half a meter to one meter, a diameter of ten to twenty centimeters;
They are located one above the other in three to seven tiers in order to save space;
They can work non-stop for up to thirty years, the record is thirty-two years.
The speed of rotation of the rotor of a gas centrifuge is such that after a power outage, it will rotate by inertia for about two months!
The boom in gas centrifuge technology is associated with the active development of nuclear energy. Nuclear plants are profit-oriented commercial enterprises and therefore need cheap fuel and therefore cheap enrichment technologies. This requirement gradually buried gaseous diffusion.
But gas centrifugation should not rest on its laurels either. Recently, more and more often you can hear about laser enrichment - a method that has been known for more than forty years. It turns out that with the help of a finely tuned laser, it is possible to selectively ionize, that is, turn uranium-235 compounds into charged particles. In this case, uranium-238 compounds are not ionized, remaining uncharged. The resulting ions can be easily separated from neutral molecules by chemical or physical means, for example, by attracting them with a magnet or a charged plate (collector).
Possible scheme of operation of the laser uranium enrichment facility
Apparently, laser enrichment is a very effective technology, but its economic performance remains a mystery. All previous attempts to move from laboratory to industrial use have been shattered by insufficient performance and short equipment life. Currently, a new attempt to create such a production is being made in the United States. But even if it is successful, the question of cost-effectiveness remains. The enrichment services market will only accept new technology if it is significantly cheaper than the existing one. But gas centrifuges have not yet reached the ceiling of their capabilities. Therefore, the immediate prospects for laser enrichment remain very vague.
There are a number of other methods of uranium enrichment: thermal diffusion, aerodynamic separation, ionic process, but they are practically not used.
When it comes to uranium enrichment technologies, it must be remembered that they open the way not only to nuclear fuel, but also to the bomb. The creation of ever more efficient and compact industries entails the threat of nuclear proliferation. In principle, the development of technology can lead to a situation where the bomb will be manufactured by states with unstable regimes, to put it mildly, or even large terrorist organizations. And if a gas diffusion or gas centrifuge plant is difficult to build unnoticed, and their launch will require the import of large volumes of characteristic materials and equipment, then laser enrichment practically guarantees secrecy. In general, the risk to the existing fragile world is increasing.
Uranium enrichment plants produce enriched uranium product (EUP) - uranium hexafluoride with the required degree of enrichment. It is placed in special containers and sent to nuclear fuel production plants. But at the same time, enrichment enterprises also produce depleted uranium hexafluoride (DUHF) with an enrichment degree of 0.3%, which is lower than that of natural uranium. In other words, it is practically pure uranium-238. Where does it come from? In essence, the beneficiation process resembles the separation of valuable minerals from waste rock. DUHF is a kind of waste rock, from which uranium-235 was withdrawn, although not completely. (One hundred percent separation of the fissile isotope from uranium-238 is unprofitable from an economic point of view). How much depleted uranium hexafluoride is formed? It depends on the required degree of uranium enrichment. For example, if it is 4.3%, as in the fuel of VVVER reactors, then ten kilograms of uranium hexafluoride, which has a natural isotopic composition (0.7% uranium-235), produces only one kilogram of OUP and nine kilograms of DUHF. In a word, quite a lot. Over the entire period of operation of the enrichment facilities, more than one and a half million tons of DUHF have been accumulated at their sites in special containers, of which about seven hundred thousand tons are in Russia. There are different attitudes towards this substance in the world, but the opinion about DUHF as a valuable strategic raw material prevails (see Chapter 7).
Fabricate - in the best sense of the word
The manufacture (fabrication) of nuclear fuel begins with the chemical transformation of the enriched uranium product into uranium dioxide. This process can be carried out in two main ways. The first of them is called "wet" technology and consists in dissolving hexafluoride in water, precipitation of sparingly soluble compounds under the action of alkali and their calcination in a hydrogen atmosphere. The second technology - "dry" - is more preferable, since it does not produce liquid radioactive waste: uranium hexafluoride is burned in a hydrogen flame.
In both cases, uranium dioxide powder is obtained, which is pressed into small tablets and sintered in furnaces at a temperature of about 1750 ° C to give them strength - after all, the tablets will have to "work" under conditions of high temperature and radiation. The tablets are then processed on grinding machines with diamond tools. This step is necessary because the dimensions of the tablet and the quality of its surface must be maintained very accurately. Errors in the manufacture of a separate pellet can lead to damage to the fuel in the reactor during its thermal expansion and, as a result, to a deterioration in the radiation situation at a nuclear power plant. Therefore, all uranium dioxide pellets are carefully controlled and after that they get into a special box, where the machine places them in tubes made of zirconium with a small admixture of niobium.
A tube loaded with pellets is called a fuel element or, for short, a fuel rod. Then, to remove corrosive gases, the fuel rod is evacuated, that is, air is “sucked out” from the tube, filled with an inert gas - the purest helium - and brewed. The last stage of the nuclear fuel fabrication process is the assembly of fuel rods into a fuel assembly (FA) using spacer grids. They are needed so that the structure is strong, and the fuel rods do not touch each other. Otherwise, at the point of contact, the shell may burn out, while the fuel will be exposed and come into contact with water, which is completely undesirable.
Sequence of operations in the production of nuclear fuel
Spacer grids
So, fuel assemblies are a "bundle" of zirconium fuel elements, inside of which there is nuclear fuel - uranium dioxide enriched in a fissile isotope. It is necessary to explain this choice of materials. In a nuclear reactor, the fuel assembly is under conditions of high temperature and a powerful flow of ionizing radiation, and is also washed from the outside with very hot pressurized water. Therefore, nuclear fuel elements must have chemical and radiation resistance, conduct heat well and expand very little when heated, otherwise a crack may occur in the fuel cladding. Uranium dioxide and zirconium meet these requirements. However, it should be recalled once again that the uranium dioxide pellets are inside the fuel elements and come into contact with water only through the fuel element cladding, but not directly. Direct interaction with the coolant is extremely undesirable and occurs only when the zirconium shells are destroyed, for example, when cracks appear in them. In this case, the radioactive fission products of uranium contained in nuclear fuel begin to dissolve in water, which leads to an increase in its radioactivity and a deterioration in the radiation situation at a nuclear power plant. For this reason, the fabrication of nuclear fuel is a complex and highly precise work that requires accuracy and constant control.
From a radiation point of view, the production of nuclear fuel does not pose a particular danger. The risk is even less than in ore mining, since the purification process removes all accompanying radioactive substances from uranium.
However, when working with enriched uranium, a critical mass can accumulate and, as a result, the self-sustaining chain reaction, which was already discussed in Chapter 2, can occur. This can happen as a result of an error, a violation of the rules of work, or even by accident. In total, sixty such accidents have been registered in the world, of which thirty-three in the USA and nineteen in the USSR/Russia. Here are two examples of domestic incidents.
July 14, 1961, Siberian Chemical Combine (enrichment). The formation of a critical mass as a result of the accumulation of uranium hexafluoride with a high degree of enrichment (22.6%) in the oil in the expansion tank of the vacuum pump. As a result of a burst of radiation that accompanied the resulting chain reaction, the operator received a significant dose of radiation and suffered radiation sickness, although in a relatively mild form.
May 15, 1997. Novosibirsk plant of chemical concentrates (production of nuclear fuel). The formation of a critical mass as a result of the accumulation of a precipitate of highly enriched (90%) uranium at the bottom of two adjacent containers for collecting solutions due to their deformation. Fortunately, the radiation doses were negligible.
What is the conclusion? Enriched uranium must be handled with extreme caution, observing all safety requirements and, as they say, "including the head", that is, calculating possible risks in advance.
In conclusion, we can give approximate parameters of fuel assemblies used at Russian NPPs with VVER-1000 reactors.
The fuel pellet is a cylinder 9 to 12 mm high and 7.6 mm in diameter. It consists of uranium dioxide, the degree of enrichment of which is in the range from 3.3 to 5.0%.
The pellets are placed in a fuel rod made of zirconium containing 1% niobium, about four meters long and 9.1 mm in diameter. The wall thickness of the fuel element is only 0.65 mm, therefore, with such a length, it requires extremely precautionary handling. The fuel element is not completely filled with pellets: the height of the layer of pellets is about 3.5 meters, and their total weight is about 1.6 kilograms, with 62 grams occupied by uranium-235.
The fuel assembly (FA) is assembled from 312 fuel rods using 12-15 spacer grids. The height of the TVS reaches almost 4.6 meters, and its mass is 760 kg. At the same time, the mass of uranium dioxide is about half a ton, the rest falls on zirconium and other metals. When viewed from above, the assembly is a hexagon with a face size of 235 millimeters. Each assembly has 19 channels for reactor control rods containing boron carbide, an element that absorbs neutrons well.
163 fuel assemblies are placed in the reactor, which corresponds to 80 tons of uranium dioxide, which is enough for 4 years of reactor operation.
Fuel assemblies for reactors of various types
Possible options
So, the most common fuel for nuclear power plants is pelleted uranium dioxide, in which uranium is enriched in the fissile isotope (uranium-235). However, there are other types of nuclear fuel.
After uranium dioxide, the most common is mixed oxide fuel, known as MOX fuel. Now, MOX fuel is mainly produced, which is a mixture of oxides of uranium and plutonium-239. This fuel makes it possible to use the excess amount of weapons-grade plutonium-239, accumulated during the "nuclear race", to generate electricity.
Uranium metal can also be used as nuclear fuel. Its advantages are high thermal conductivity and the maximum concentration of fissile nuclei - there are simply no other elements in the fuel. At the same time, uranium, as a metal, has poorer radiation, chemical, and heat resistance than dioxide, so it is rarely used in its pure form. To improve the parameters of metallic fuel, some molybdenum, aluminum, silicon, and zirconium are added to uranium. Today, metallic uranium and its alloys are used only in research reactors.
Instead of uranium dioxide, it is possible to use uranium nitride, that is, its combination with nitrogen. Nitride fuel has a higher thermal conductivity compared to dioxide, and a comparable melting point (2855 o C). Uranium nitride is considered a promising fuel for the latest reactors. In our country, nitride fuel is given the closest attention, as it is planned to be used in the next generation of fast neutron reactors.
Uranium is able to form compounds with carbon - carbides. The possibility of using carbides as fuel for reactors was intensively studied in the 1960s and 1970s. However, in the recent period, interest in this type of fuel has re-emerged, associated with the development of plate fuel elements and microfuel elements. The positive features of carbides are good thermal conductivity, high melting point, high hardness, chemical and thermal stability, and compatibility with ceramic coatings, which is especially important for microfuels. Uranium carbide fuel may be the best option for certain types of next-generation reactors, in particular for gas-cooled fast reactors.
But still, the vast majority of reactors on Earth still operate on nuclear fuel made from uranium dioxide. The power of tradition, so to speak.
Russian fuel cycle
Now, having familiarized ourselves with the peculiarities of the operation of mining and processing industries, it is worth taking a quick look at the history and current state of our domestic fuel cycle. You need to start, of course, with the extraction of uranium.
At first, uranium ores were of interest to domestic scientists only as a source of radium. In 1900, Professor I.A. Antipov made a report at a meeting of the St. Petersburg Mineralogical Society about the discovery of the uranium mineral in samples brought from Fergana, from the Tyuya-Muyun mountain range. Later, this mineral was named tyuyamunite. In 1904, exploration work began at this deposit, in 1908 a pilot plant for processing uranium ore was built in St. Petersburg, and in 1913 an international joint-stock company for the extraction of Tuyamuyun radium was established.
When the First World War began, work at the mine practically ceased, and only in 1922 an expedition consisting of eight specialists was sent to Tyuya-Muyun. In the same 1922, in difficult post-revolutionary conditions, surrounded by bands of Basmachi, it was possible to re-establish industrial ore mining. It continued until 1936, when abundant groundwater at a depth of two hundred meters interrupted the development of the deposit. However, this problem did not become critical, since the extraction of radium was established at the "Water industry" on the Ukhta River - the radioactive metal was extracted from underground salt water. Uranium itself in those years was of little interest to anyone, since it was practically not used in industry.
A new surge of interest in uranium deposits occurred in the early 1940s, when the USSR faced the need to respond to the nuclear threat emanating from the United States, that is, when the need arose to create domestic nuclear weapons.
Uranium for the first Soviet atomic bomb was literally collected bit by bit throughout the country and beyond. In 1943, uranium mining began at the tiny, by modern standards, Taboshar mine in Tajikistan, with a capacity of only 4 tons of uranium salts per year. Moreover, according to the memoirs of P.Ya. Antropov, the first Minister of Geology of the USSR, “uranium ore was transported for processing along the mountain paths of the Pamirs in sacks on donkeys and camels. There were no roads or proper equipment then.
In 1944-1945, as Europe was liberated from the Nazis, the USSR gained access to uranium ore from the Goten deposit in Bulgaria, the Yachimov mines in Czechoslovakia, and the mines of German Saxony. In addition, in 1946, the Tyuya-Muyunsky mine was re-launched, but it did not make a special contribution to the common cause.
In the 1950s, the Lermontov Almaz Production Association began mining uranium at mines in the Beshtau and Byk mountains (Stavropol Territory). At the same time, they began to develop deposits in South Kazakhstan and Central Asia.
After 1991, most of the developed fields ended up outside Russia, in independent states. From that moment on, the main uranium mining is carried out by the mine method at the Priargunsky Production Mining and Chemical Association (Transbaikal Territory). In addition, two enterprises using the technology of borehole in-situ leaching are gradually gaining strength - Khiagda (Republic of Buryatia) and Dalur (Kurgan region). Production facilities are being designed in Yakutia. There are also promising regions for production - Transbaikal, West Siberian, North European ...
In terms of explored uranium reserves, Russia ranks third in the world.
Russian uranium mining enterprises are managed by ARMZ Uranium Holding (www.armz.ru), owned by Rosatom, but the State Corporation also has foreign assets controlled by the international company Uranium One Inc. (www.uranium1.com). Thanks to the activities of these two organizations, Rosatom has reached the third place in the world in the production of uranium compounds.
The situation in the world market for the production of natural uranium (2014)
The baton from mining enterprises is taken over by a whole complex of productions for refining, conversion and enrichment of uranium, as well as for the fabrication of nuclear fuel. Most of them come from the period and the fifties of the last century - the time of active accumulation of nuclear weapons. Today they work for a purely peaceful industry - nuclear energy, and provide their services to foreign companies.
There are four enrichment plants in Russia, some of them also carry out operations for the final purification (refining) and fluorination (conversion) of uranium compounds.
The first gas diffusion plant for uranium enrichment D-1 in Sverdlovsk-44 started operating in November 1949. At first, its products had to be further enriched at the SU-20 unit of the future Elektrokhimpribor plant in Sverdlovsk-45 (Lesnoy), but after a couple of years, D-1 began to cope on its own and began to grow. And since 1967, the replacement of diffusion cascades by cascades of centrifuges began. Today, on the site of the dismantled D-1, there is the world's largest uranium enrichment enterprise - the Ural Electrochemical Plant (Novouralsk, Sverdlovsk Region).
In 1953, the future Siberian Chemical Plant (Seversk, Tomsk Region) began work in Tomsk-7, which, since 1973, began to gradually switch to gas centrifuge technology. The first enriched uranium from the Angarsk electrolysis chemical plant (Angarsk, Irkutsk region) was obtained in 1957, and the replacement of diffusion apparatuses with centrifuges started in 1985. Finally, 1962 became the year of launching the Electrochemical Plant in Krasnoyarsk-45 (now Zelenogorsk, Krasnoyarsk Territory). A couple of years later, the first centrifuges were installed there.
This brief reference, of course, does not reflect the realities of that difficult era. Although by the secret, "numbered" names of closed cities and by the vague names of plants, one can understand that the Soviet Union carefully kept its secrets of enrichment. However, the locations of the main production facilities became known to American intelligence. But the active transition to gas centrifuge technology, she, as they say, missed it. Perhaps this was the reason for some complacency of our competitors: not knowing that a more productive and efficient technology was being introduced in the USSR, the States adhered to the initially chosen method - gaseous diffusion. Obviously, the current situation played into the hands of the Soviet Union and made it possible to quickly achieve nuclear parity. At the same time, the pioneering developments of Soviet scientists and engineers to create high-performance gas centrifuges did not go to waste, bringing Russia to a leading position in the world market for uranium enrichment and centrifuge production.
The enriched uranium product from four combines is supplied to the Machine-Building Plant (Elektrostal, Moscow Region) and the Novosibirsk Chemical Concentrates Plant (Novosibirsk, the same-named Region), where a full cycle of nuclear fuel production is carried out. Zirconium for fuel rods and other structural materials for fuel assemblies are supplied by the Chepetsky Mechanical Plant (Glazov, Udmurt Republic), the only enterprise in Russia and the third in the world to manufacture products from zirconium.
The manufactured fuel assemblies are delivered to Russian and foreign nuclear power plants, and are also used in reactors for other purposes.
Enterprises for refining, conversion and enrichment of uranium, fabrication of nuclear fuel, production of gas centrifuges, as well as design and research organizations are united as part of the TVEL Fuel Company of Rosatom (www.tvel.ru).
As a result of many years of successful work of this company and its enterprises, Rosatom confidently tops the list of the largest service providers in the field of uranium enrichment (36% of the world market).
There is a nuclear fuel bank in Angarsk - a guaranteed reserve that can be purchased by a country that for some reason is deprived of the opportunity to purchase uranium on the free market. From this stock, it will be able to produce fresh nuclear fuel and ensure the uninterrupted operation of its nuclear power industry.
Rosatom's share in the global nuclear fuel market is 17%, due to which every sixth power reactor on Earth is loaded with fuel of the TVEL brand. Deliveries go to Hungary, Slovakia, Czech Republic, Bulgaria, Ukraine, Armenia, Finland, India and China.
Above - the world market for uranium enrichment (2015), below - the world market for fuel fabrication (2015)
Open or closed?
It may be noted that this chapter did not cover the production of nuclear fuel for research reactors, as well as reactors installed on nuclear submarines and icebreakers. The entire discussion was devoted to nuclear fuel used in nuclear power plants. However, this was not done by accident. The fact is that there are simply no fundamental differences between the sequence of fuel production for nuclear power plants and, for example, nuclear submarines. Of course, there may be deviations in technology related to the specifics of ship and research reactors. For example, the former should be small in size and, at the same time, quite powerful - this is a completely natural requirement for an icebreaker and, moreover, a maneuverable nuclear submarine. The necessary indicators can be achieved by increasing the enrichment of uranium, that is, by increasing the concentration of fissile nuclei - then less fuel will be needed. This is exactly what they do: the degree of enrichment of uranium used as fuel for ship reactors is in the region of 40% (depending on the project, it can vary from 20 to 90%). In research reactors, the usual requirement is to achieve maximum neutron flux, and the number of neutrons in the reactor is also directly related to the number of fissile nuclei. Therefore, in installations intended for scientific research, highly enriched uranium with a much higher content of uranium-235 is sometimes used than in the fuel of nuclear power reactors. But the enrichment technology does not change from this.
The design of the reactor can determine the chemical composition of the fuel and the material from which the fuel rod is made. Currently, the main chemical form of fuel is uranium dioxide. As for the fuel elements, they are predominantly zirconium, but, for example, stainless steel fuel elements are produced for the BN-600 fast neutron reactor. This is due to the use of liquid sodium as a coolant in BN reactors, in which zirconium is destroyed (corroded) faster than stainless steel. Nevertheless, the essence of the nuclear fuel fabrication process remains the same - uranium dioxide powder is synthesized from the enriched uranium product, which is pressed into pellets and sintered, the pellets are placed in fuel rods, and the fuel rods are assembled into fuel assemblies (FA).
Moreover, if we consider the nuclear fuel cycles of various countries, it turns out, for example, that in Russia uranium compounds are directly fluorinated with molecular fluorine during conversion, while abroad they are first treated with hydrofluoric acid and only then with fluorine. The difference can be found in the chemical composition of solutions for "opening" the ore, sorbents and extractants; the parameters of the processes may differ ... But the scheme of the nuclear fuel cycle does not change from this. The fundamental difference lies only between its open (open) and closed (closed) versions: in the first case, after “working” at a nuclear power plant, the fuel is simply isolated from the environment in a deep burial ground, and in the latter, it is processed with the extraction of valuable components (see chapter 7). Russia is one of the few countries implementing a closed cycle.
An example of a closed fuel cycle indicating the role of TVEL Fuel Company of Rosatom